Troponin I phosphorylation enhances crossbridge kinetics during beta-adrenergic stimulation in rat cardiac tissue
- PMID: 12154188
- PMCID: PMC2290461
- DOI: 10.1113/jphysiol.2002.022707
Troponin I phosphorylation enhances crossbridge kinetics during beta-adrenergic stimulation in rat cardiac tissue
Abstract
Inotropic agents that increase the intracellular levels of cAMP have been shown to increase crossbridge turnover kinetics in intact rat ventricular muscle, as measured by the parameter f(min) (the frequency at which dynamic stiffness is minimum). These agents are also known to increase the level of phosphorylation of two candidate myofibrillar proteins: myosin binding protein C (MyBPC) and Troponin I (TnI), but have no effect on myosin light chain 2 phosphorylation (MyLC2). The aim of this study was to investigate whether the phosphorylation of TnI and/or MyBPC was responsible for the increase in crossbridge cycling kinetics (as captured by f(min)) seen with the elevation of cAMP within cardiac tissue. Using barium-activated intact rat papillary muscle, we investigated the actions of isobutylmethylxanthine (IBMX), an inhibitor of cAMP-dependent phosphatase, which simulates the action of beta-adrenergic agents, and the chemical phosphatase 2,3-butanedione monoxime (BDM), which has been shown to dephosphorylate a number of contractile proteins. The presence of 0.6 mM IBMX approximately doubled the f(min) value of intact rat papillary muscle. This action was unaffected by the addition of BDM. In the presence of IBMX and BDM, the level of phosphorylation of MyBPC was unchanged, that of MyLC2 was reduced to 60 % of control, yet that of TnI was markedly increased (to 30 % above control levels). We conclude that TnI phosphorylation, mediated by cAMP-dependent protein kinase A, is the molecular basis for the enhanced crossbridge cycling seen during beta-adrenergic stimulation of the heart.
Figures
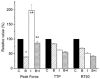
 , n = 10) sampled at 5 min after addition to muscle bath, IBMX (I, □, n = 8) sampled at 3.5 min, BDM + IBMX (B + I,
, n = 10) sampled at 5 min after addition to muscle bath, IBMX (I, □, n = 8) sampled at 3.5 min, BDM + IBMX (B + I,  , n = 20) sampled at plateau, typically 5 min after addition of the second agent. All timing parameters are significantly different from control and between treatments. For peak isometric tension: a significantly different when compared to control value; b significantly different when compared to BDM value; c significantly different when compared to IBMX value.
, n = 20) sampled at plateau, typically 5 min after addition of the second agent. All timing parameters are significantly different from control and between treatments. For peak isometric tension: a significantly different when compared to control value; b significantly different when compared to BDM value; c significantly different when compared to IBMX value.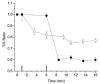
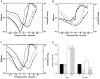
 , n = 12), IBMX (I, □, n = 8) and a combination of BDM and IBMX (B + I,
, n = 12), IBMX (I, □, n = 8) and a combination of BDM and IBMX (B + I,  , n = 15). All values are normalised to the control (C, ▪). The control fmin value was 2.1 ± 0.7 Hz (n = 20). aSignificantly different when compared to the control value; bsignificantly different from BDM value; csignificantly different from IBMX value.
, n = 15). All values are normalised to the control (C, ▪). The control fmin value was 2.1 ± 0.7 Hz (n = 20). aSignificantly different when compared to the control value; bsignificantly different from BDM value; csignificantly different from IBMX value.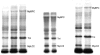
 , n = 4), IBMX (I, □, n = 4) and BDM + IBMX (B + I,
, n = 4), IBMX (I, □, n = 4) and BDM + IBMX (B + I,  , n = 5). MyBPC, Myosin binding protein C; TnI, troponin I; MLC2, myosin light chain 2. a Significantly different when compared to control value; b significantly different from BDM value; c significantly different from IBMX value; d significantly different from BDM + IBMX value.
, n = 5). MyBPC, Myosin binding protein C; TnI, troponin I; MLC2, myosin light chain 2. a Significantly different when compared to control value; b significantly different from BDM value; c significantly different from IBMX value; d significantly different from BDM + IBMX value.Similar articles
-
Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle.Circ Res. 2001 May 25;88(10):1059-65. doi: 10.1161/hh1001.091640. Circ Res. 2001. PMID: 11375276
-
Mechanisms of the contractile effects of 2,3-butanedione-monoxime in the mammalian heart.Naunyn Schmiedebergs Arch Pharmacol. 1996 Oct;354(4):431-6. doi: 10.1007/BF00168433. Naunyn Schmiedebergs Arch Pharmacol. 1996. PMID: 8897445
-
The contributions of cardiac myosin binding protein C and troponin I phosphorylation to β-adrenergic enhancement of in vivo cardiac function.J Physiol. 2016 Feb 1;594(3):669-86. doi: 10.1113/JP270959. J Physiol. 2016. PMID: 26635197 Free PMC article.
-
Cardiac contractility: modulation of myofibrillar calcium sensitivity by beta-adrenergic stimulation.Isr J Med Sci. 1997 Jan;33(1):1-7. Isr J Med Sci. 1997. PMID: 9203510 Review.
-
Regulation of cardiac contractile proteins. Correlations between physiology and biochemistry.Circ Res. 1984 Nov;55(5):565-74. doi: 10.1161/01.res.55.5.565. Circ Res. 1984. PMID: 6091939 Review.
Cited by
-
Insights into length-dependent regulation of cardiac cross-bridge cycling kinetics in human myocardium.Arch Biochem Biophys. 2016 Jul 1;601:48-55. doi: 10.1016/j.abb.2016.02.005. Epub 2016 Feb 18. Arch Biochem Biophys. 2016. PMID: 26854725 Free PMC article.
-
CaMKII-dependent myofilament Ca2+ desensitization contributes to the frequency-dependent acceleration of relaxation.Cell Calcium. 2015 Nov;58(5):489-99. doi: 10.1016/j.ceca.2015.08.001. Epub 2015 Aug 7. Cell Calcium. 2015. PMID: 26297240 Free PMC article.
-
Cardiac myosin isoforms exhibit differential rates of MgADP release and MgATP binding detected by myocardial viscoelasticity.J Mol Cell Cardiol. 2013 Jan;54:1-8. doi: 10.1016/j.yjmcc.2012.10.010. Epub 2012 Oct 30. J Mol Cell Cardiol. 2013. PMID: 23123290 Free PMC article.
-
Cardiac troponin structure-function and the influence of hypertrophic cardiomyopathy associated mutations on modulation of contractility.Arch Biochem Biophys. 2016 Jul 1;601:11-21. doi: 10.1016/j.abb.2016.02.004. Epub 2016 Feb 4. Arch Biochem Biophys. 2016. PMID: 26851561 Free PMC article. Review.
-
Tri-modal regulation of cardiac muscle relaxation; intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics.Biophys Rev. 2014 Dec;6(3-4):273-289. doi: 10.1007/s12551-014-0143-5. Epub 2014 Jul 17. Biophys Rev. 2014. PMID: 28510030 Free PMC article.
References
-
- Allen TJ, Chapman RA. The effect of a chemical phosphatase on single calcium channels and the inactivation of whole-cell calcium current from isolated guinea-pig ventricular myocytes. Pflügers Archiv. 1995;430:68–80. - PubMed
-
- Blaineau S, Jacquemond V, Allard B, Amsellem J, Moutin MJ, Rougier O. Inward barium current and excitation-contraction coupling in frog twitch muscle fibres. Journal of Muscle Research and Cell Motility. 1993;14:158–166. - PubMed
-
- Blanchard E, Seidman C, Seidman JG, Lewinter M, Maughan D. Altered crossbridge kinetics in the alpha MHC403/+ mouse model of familial hypertrophic cardiomyopathy. Circulation Research. 1999;84:475–483. - PubMed
-
- Coulombe A, Lefevre A, Deroubaix E, Thuringer D, Coraboeuf E. Effect of 2,3-butanedione monoxime on slow inward and transient outward currents in rat ventricular myocytes. Journal of Molecular and Cellular Cardiology. 1990;22:921–932. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

