Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia
- PMID: 12154229
- PMCID: PMC123277
- DOI: 10.1073/pnas.162371899
Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia
Abstract
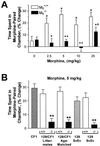
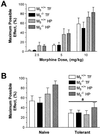
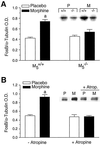
Little is known about the physiological roles of the M5 muscarinic receptor, the last member of the muscarinic receptor family (M1-M5) to be cloned. In the brain, the M5 receptor subtype is preferentially expressed by dopaminergic neurons of the substantia nigra and the ventral tegmental area. Dopaminergic neurons located in the ventral tegmental area are known to play important roles in mediating both the rewarding effects of opiates and other drugs of abuse and the manifestations of opiate/drug withdrawal symptoms. We therefore speculated that acetylcholine-dependent activation of M5 receptors might modulate the manifestations of opiate reward and withdrawal. This hypothesis was tested in a series of behavioral, biochemical, and neurochemical studies using M5 receptor-deficient mice (M5-/- mice) as novel experimental tools. We found that the rewarding effects of morphine, as measured in the conditioned place preference paradigm, were substantially reduced in M5-/- mice. Furthermore, both the somatic and affective components of naloxone-induced morphine withdrawal symptoms were significantly attenuated in M5-/- mice. In contrast, the analgesic efficacy of morphine and the development of tolerance to the analgesic effects of morphine remained unaltered by the lack of M5 receptors. The finding that M5 receptor activity modulates both morphine reward and withdrawal processes suggests that M5 receptors may represent a novel target for the treatment of opiate addiction.
Figures




References
-
- Bonner T. I., Young, A. C., Brann, M. R. & Buckley, N. J. (1988) Neuron 1, 403-410. - PubMed
-
- Liao C. F., Themmen, A. P., Joho, R., Barberis, C., Birnbaumer, M. & Birnbaumer, L. (1989) J. Biol. Chem. 264, 7328-7337. - PubMed
-
- Wess J. (1996) Crit. Rev. Neurobiol. 10, 69-99. - PubMed
-
- Caulfield M. P. & Birdsall, N. J. M. (1998) Pharmacol. Rev. 50, 279-290. - PubMed
-
- Reever C. M., Ferrari-DiLeo, G. & Flynn, D. D. (1997) Life Sci. 60, 1105-1112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

