Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis
- PMID: 12163371
- PMCID: PMC1850724
- DOI: 10.1016/S0002-9440(10)64202-2
Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis
Abstract
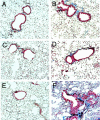
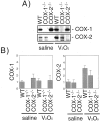
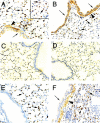
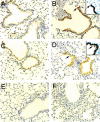
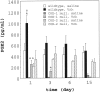
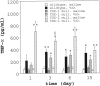
The cyclooxygenase (COX)-2 enzyme has been implicated as an important mediator of pulmonary fibrosis. In this study, the lung fibrotic responses were investigated in COX-1 or COX-2-deficient (-/-) mice following vanadium pentoxide (V(2)O(5)) exposure. Lung histology was normal in saline-instilled wild-type and COX-deficient mice. COX-2(-/-), but not COX-1(-/-) or wild-type mice, exhibited severe inflammatory responses by 3 days following V(2)O(5) exposure and developed pulmonary fibrosis 2 weeks post-V(2)O(5) exposure. Western blot analysis and immunohistochemistry showed that COX-1 protein was present in type 2 epithelial cells, bronchial epithelial cells, and airway smooth muscle cells of saline or V(2)O(5)-exposed wild-type and COX-2(-/-) mice. COX-2 protein was present in Clara cells of wild-type and COX-1(-/-) terminal bronchioles and was strongly induced 24 hours after V(2)O(5) exposure. Prostaglandin (PG) E(2) levels in the bronchoalveolar lavage (BAL) fluid from wild-type and COX-1(-/-) mice were significantly up-regulated by V(2)O(5) exposure within 24 hours, whereas PGE(2) was not up-regulated in COX-2(-/-) BAL fluid. Tumor necrosis factor-alpha was elevated in the BAL fluid from all genotypes after V(2)O(5) exposure, but was significantly and chronically elevated in the BAL fluid from COX-2(-/-) mice above wild-type or COX-1(-/-) mice. These findings indicate that the COX-2 enzyme is protective against pulmonary fibrogenesis, and we suggest that COX-2 generation of PGE(2) is an important factor in resolving inflammation.
Figures


References
-
- Smith WL, Dewitt DL: Prostaglandin endoperoxidase H synthases-1 and -2. Adv Immunol 1996, 62:167-215 - PubMed
-
- Vancheri C, Sortino MA, Tomaselli V, Mastruzzo C, Condorelli F, Bellistri G, Pistorio MP, Canonico PL, Crimi N: Different expression of TNF-α receptors and prostaglandin-E2 production in normal and fibrotic lung fibroblasts. Am J Respir Cell Mol Biol 2000, 22:628-634 - PubMed
-
- Boyle JE, Lindroos PM, Rice AB, Zhang L, Zeldin DC, Bonner JC: Prostaglanin-E2 counteracts interleukin-1β-stimulated up-regulation of platelet-derived growth factor α-receptor on rat pulmonary myofibroblasts. Am J Respir Cell Mol Biol 1999, 20:433-440 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

