Gamma-glutamyl leukotrienase, a novel endothelial membrane protein, is specifically responsible for leukotriene D(4) formation in vivo
- PMID: 12163373
- PMCID: PMC1850737
- DOI: 10.1016/s0002-9440(10)64204-6
Gamma-glutamyl leukotrienase, a novel endothelial membrane protein, is specifically responsible for leukotriene D(4) formation in vivo
Abstract
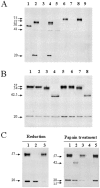
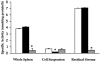
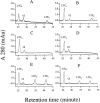
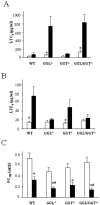
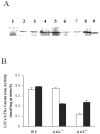
The metabolism of cysteinyl leukotrienes in vivo and the pathophysiological effects of individual cysteinyl leukotrienes are primarily unknown. Recently we identified an additional member of the gamma-glutamyl transpeptidase (GGT) family, gamma-glutamyl leukotrienase (GGL), and developed mice deficient in this enzyme. Here we show that in vivo GGL, and not GGT as previously believed, is primarily responsible for conversion of leukotriene C(4) to leukotriene D(4), the most potent of the cysteinyl leukotrienes and the immediate precursor of leukotriene E(4). GGL is a glycoprotein consisting of two polypeptide chains encoded by one gene and is attached at the amino terminus of the heavy chain to endothelial cell membranes. In mice it localizes to capillaries and sinusoids in most organs and in lung to larger vessels as well. In contrast to wild-type and GGT-deficient mice, GGL-deficient mice do not form leukotriene D(4) in vivo either in blood when exogenous leukotriene C(4) is administered intravenously or in bronchoalveolar lavage fluid of Aspergillus fumigatus extract-induced experimental asthma. Further, GGL-deficient mice show leukotriene C(4) accumulation and significantly more airway hyperreponsiveness than wild-type mice in the experimental asthma, and induction of asthma results in increased GGL protein levels and enzymatic activity. Thus GGL plays an important role in leukotriene D(4) synthesis in vivo and in inflammatory processes.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

