Relationship of sialyl-Lewis(x/a) underexpression and E-cadherin overexpression in the lymphovascular embolus of inflammatory breast carcinoma
- PMID: 12163386
- PMCID: PMC1850721
- DOI: 10.1016/S0002-9440(10)64217-4
Relationship of sialyl-Lewis(x/a) underexpression and E-cadherin overexpression in the lymphovascular embolus of inflammatory breast carcinoma
Abstract
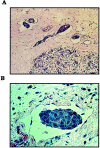
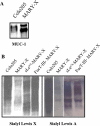
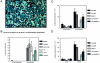
Inflammatory breast carcinoma (IBC) is characterized by florid tumor emboli within lymphovascular spaces called lymphovascular invasion. These emboli have a unique microscopic appearance of compact clumps of tumor cells retracted away from the surrounding endothelial cell layer. Using a human SCID model of IBC (MARY-X), we, in previous studies, demonstrated that the tumor cell embolus (IBC spheroid) forms on the basis of an intact and overexpressed E-cadherin/alpha,beta-catenin axis that mediates tumor cell-tumor cell adhesion. In the present study we examine the mechanism behind the apparent lack of binding of the tumor embolus to the surrounding endothelium. We find that this lack of tumor cell binding is because of markedly decreased sialyl-Lewis(x/a) (sLe(x/a)) carbohydrate ligand-binding epitopes on its overexpressed MUC1 and other surface molecules that bind endothelial E-selectin. Decreased sLe(x/a) is because of decreased alpha3/4-fucosyltransferase activity in MARY-X. The decreased sLe(x/a) fail to confer electrostatic repulsions between tumor cells, which further contributes to the compactness of the MARY-X spheroid by allowing the E-cadherin homodimeric interactions to go unopposed. MARY-X spheroids were retrovirally transfected with FucT-III cDNA, significantly raising their levels of fucosyltransferase activity and surface sLe(x/a). In parallel experiments, enzymatic transfers with a milk alpha1,3-fucosyltransferase and an alpha2,3-sialyltransferase (ST3GalIV) were performed on the MARY-X spheroids and increased surface sLe(x/a). The addition of sLe(x/a) by either manipulation caused disadherence of the MARY-X spheroids and the disruption of the E-cadherin homodimers mediating cell adhesion. Our findings support the cooperative relationship of sLe(x/a) underexpression and E-cadherin overexpression in the genesis of the lymphovascular embolus of IBC.
Figures




References
-
- Palangie T, Mosseri B, Mihura J, Campana F, Beuzeboc P, Dorval T, Garcia-Giralt E, Jouve M, Scholl S, Asselain B, Pouillart P: Prognostic factors in inflammatory breast cancer and therapeutic implications. Eur J Cancer 1994, 30A:921-927 - PubMed
-
- Levine PH, Steinhorn SC, Ries LG, Aron JL: Inflammatory breast cancer: the experience of the surveillance, epidemiology, and end results (SEER) program. J Natl Cancer Inst 1985, 74:291-297 - PubMed
-
- Quigley JP, Armstrong PB: Tumor cell intravasation alu-cidated: the chick embryo opens the window. Cell 1998, 94:281-284 - PubMed
-
- Alpaugh ML, Tomlinson JS, Shao ZM, Barsky SH: A novel human xenograft model of inflammatory breast cancer. Cancer Res 1999, 59:5079-5084 - PubMed
-
- Tomlinson JS, Alpaugh ML, Barsky SH: An intact overexpressed E-cadherin/α, β-catenin axis characterizes the lymphovascular emboli of inflammatory breast cancer. Cancer Res 2001, 61:5231-5241 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

