L-type calcium channel-mediated plateau potentials in barrelette cells during structural plasticity
- PMID: 12163531
- PMCID: PMC3686508
- DOI: 10.1152/jn.2002.88.2.794
L-type calcium channel-mediated plateau potentials in barrelette cells during structural plasticity
Abstract
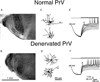
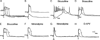
Development and maintenance of whisker-specific patterns along the rodent trigeminal pathway depends on an intact sensory periphery during the sensitive/critical period in development. Barrelette cells of the brain stem trigeminal nuclei are the first set of neurons to develop whisker-specific patterns. Those in the principal sensory nucleus (PrV) relay these patterns to the ventrobasal thalamus, and consequently, to the somatosensory cortex. Thus PrV barrelette cells are among the first group of central neurons susceptible to the effects of peripheral damage. Previously we showed that membrane properties of barrelette cells are distinct as early as postnatal day 1 (PND 1) and remain unchanged following peripheral denervation in newborn rat pups (Lo and Erzurumlu 2001). In the present study, we investigated the changes in synaptic transmission. In barrelette cells of normal PND 1 rats, weak stimulation of the trigeminal tract (TrV) that was subthreshold for inducing Na(+) spikes evoked an excitatory postsynaptic potential-inhibitory postsynaptic potential (EPSP-IPSP) sequence that was similar to the responses seen in older rats (Lo et al. 1999). Infraorbital nerve transection at birth did not alter excitatory and inhibitory synaptic connections of the barrelette cells. These observations suggested that local neuronal circuits are already established in PrV at birth and remain intact after deafferentation. Strong stimulation of the TrV induced a sustained depolarization (plateau potential) in denervated but not in normal barrelette neurons. The plateau potential was distinct from the EPSP-IPSP sequence by 1) a sustained (>80 ms) depolarization above -40 mV; 2) a slow decline slope (<0.1 mV/ms); 3) partially or totally inactivated Na(+) spikes on the plateau; and 4) a termination by a steep decay (>1 mV/ms) to a hyperpolarizing membrane level. The plateau potential was mediated by L-type Ca(2+) channels and triggered by a N-methyl-D-aspartate (NMDA) receptor-mediated EPSP. gamma-aminobutyric acid-A (GABA(A)) receptor-mediated IPSP dynamically regulated the latency and duration of the plateau potential. These results indicate that after neonatal peripheral damage, central trigeminal inputs cause a large and long-lasting Ca(2+) influx through L-type Ca(2+) channels in barrelette neurons. Increased Ca(2+) entry may play a key role in injury-induced structural remodeling, and/or transsynaptic cell death.
Figures


References
-
- Arends JJ, Jacquin MF. Lucifer Yellow staining in fixed brain slices: optimal methods and compatibility with somatotopic markers in neonatal brain. J Neurosci Methods. 1993;50:321–339. - PubMed
-
- Ashwell KW, Waite PME. Cell death in the developing trigeminal nuclear complex of the rat. Dev Brain Res. 1991;63:291–295. - PubMed
-
- Bates CA, Killackey HP. The organization of the neonatal rat’s brainstem trigeminal complex and its role in the formation of central patterns. J Comp Neurol. 1985;240:265–287. - PubMed
-
- Blanton MG, Turco JJL, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. - PubMed
-
- Campbell NC, Hesslow G. Plateau potentials evoked by climbing-fibre stimulation are restricted to the Purkinje cell dendrites of the cat. Neurosci Lett. 1984;45:187–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

