Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation
- PMID: 12163567
- PMCID: PMC2193938
- DOI: 10.1084/jem.20020399
Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation
Abstract
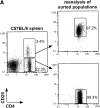
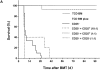
Acute graft-versus-host disease (aGVHD) is still a major obstacle in clinical allogeneic bone marrow (BM) transplantation. CD4(+)CD25(+) regulatory T (T(reg)) cells have recently been shown to suppress proliferative responses of CD4(+)CD25(-) T cells to alloantigenic stimulation in vitro and are required for ex vivo tolerization of donor T cells, which results in their reduced potential to induce aGVHD. Here we show that CD4(+)CD25(+) T cells isolated from the spleen or BM of donor C57BL/6 (H-2(b)) mice that have not been tolerized are still potent inhibitors of the alloresponse in vitro and of lethal aGVHD induced by C57BL/6 CD4(+)CD25(-) T cells in irradiated BALB/c (H-2(d)) hosts in vivo. The addition of the CD4(+)CD25(+) T(reg) cells at a 1:1 ratio with responder/inducer CD4(+)CD25(-) T cells resulted in a >90% inhibition of the mixed leukocyte reaction and marked protection from lethal GVHD. This protective effect depended in part on the ability of the transferred CD4(+)CD25(+) T cells to secrete interleukin 10 and occurred if the T(reg) cells were of donor, but not host, origin. Our results demonstrate that the balance of donor-type CD4(+)CD25(+) T(reg) and conventional CD4(+)CD25(-) T cells can determine the outcome of aGVHD.
Figures










References
-
- Klingebiel, T., and P.G. Schlegel. 1998. GVHD: overview on pathophysiology, incidence, clinical and biological features. Bone Marrow Transplant. 21:S45–S49. - PubMed
-
- Deeg, H.J., and R. Storb. 1984. Graft-versus-host disease: pathophysiological and clinical aspects. Annu. Rev. Med. 35:11–24. - PubMed
-
- Snider, D., and H. Liang. 2001. Early intestinal Th1 inflammation and mucosal T cell recruitment during acute graft-versus-host reaction. J. Immunol. 166:5991–5999. - PubMed
-
- Ferrara, J.L., R. Levy, and N.J. Chao. 1999. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol. Blood Marrow Transplant. 5:347–356. - PubMed
-
- Hill, G.R., and J.L. Ferrara. 2000. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 95:2754–2759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

