Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling
- PMID: 12163593
- PMCID: PMC136986
- DOI: 10.1128/jvi.76.17.8729-8736.2002
Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling
Abstract
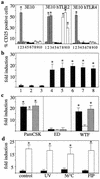
Pattern recognition via Toll-like receptors (TLR) by antigen-presenting cells is an important element of innate immunity. We report that wild-type measles virus but not vaccine strains activate cells via both human and murine TLR2, and this is a property of the hemagglutinin (H) protein. The ability to activate cells via TLR2 by wild-type MV H protein is abolished by mutation of a single amino acid, asparagine at position 481 to tyrosine, as is found in attenuated strains, which is important for interaction with CD46, the receptor for these strains. TLR2 activation by MV wild-type H protein stimulates induction of proinflammatory cytokines such as interleukin-6 (IL-6) in human monocytic cells and surface expression of CD150, the receptor for all MV strains. Confirming the specificity of this interaction, wild-type H protein did not induce IL-6 release in macrophages from TLR2-/- mice. Thus, the unique property of MV wild-type strains to activate TLR2-dependent signals might essentially contribute not only to immune activation but also to viral spread and pathogenicity by upregulating the MV receptor on monocytes.
Figures

References
-
- Aarden, L. A., E. R. De Groot, O. L. Schaap, and P. M. Lansdorp. 1987. Production of hybridoma growth factor by human monocytes. Eur. J. Immunol. 17:1411-1417. - PubMed
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. - PubMed
-
- Alexopoulou, L., A. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. - PubMed
-
- Arbibe, L., J. P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P. J. Godowski, R. J. Ulevitch, and U. G. Knaus. 2000. Toll-like receptor 2-mediated NF-κB activation requires a RacI-dependent pathway. Nat. Immunol. 1:533-540. - PubMed
-
- Astier, A., M. C. Trescol-Biemont, O. Azocar, B. Lamouille, and C. Rabourdin-Combe. 2000. CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J. Immunol. 164:6091-6095. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

