Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1
- PMID: 12163607
- PMCID: PMC136973
- DOI: 10.1128/jvi.76.17.8875-8889.2002
Stabilization of the soluble, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1
Abstract
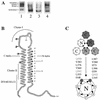
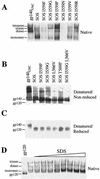
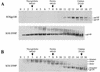
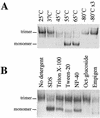
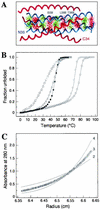
The envelope glycoprotein (Env) complex of human immunodeficiency virus type 1 has evolved a structure that is minimally immunogenic while retaining its natural function of receptor-mediated virus-cell fusion. The Env complex is trimeric; its six individual subunits (three gp120 and three gp41 subunits) are associated by relatively weak, noncovalent interactions. The induction of neutralizing antibodies after vaccination with individual Env subunits has proven very difficult, probably because they are inadequate mimics of the native complex. Our hypothesis is that a stable form of the Env complex, perhaps with additional modifications to rationally alter its antigenic structure, may be a better immunogen than the individual subunits. A soluble form of Env, SOS gp140, can be made that has gp120 stably linked to the gp41 ectodomain by an intermolecular disulfide bond. This protein is fully cleaved at the proteolysis site between gp120 and gp41. However, the gp41-gp41 interactions in SOS gp140 are too weak to maintain the protein in a trimeric configuration. Consequently, purified SOS gp140 is a monomer (N. Schülke, M. S. Vesanen, R. W. Sanders, P. Zhu, D. J. Anselma, A. R. Villa, P. W. H. I. Parren, J. M. Binley, K. H. Roux, P. J. Maddon, J. P. Moore, and W. C. Olson, J. Virol. 76:7760-7776, 2002). Here we describe modifications of SOS gp140 that increase its trimer stability. A variant SOS gp140, designated SOSIP gp140, contains an isoleucine-to-proline substitution at position 559 in the N-terminal heptad repeat region of gp41. This protein is fully cleaved, has favorable antigenic properties, and is predominantly trimeric. SOSIP gp140 trimers are noncovalently associated and can be partially purified by gel filtration chromatography. These gp140 trimers are dissociated into monomers by anionic detergents or heat but are relatively resistant to nonionic detergents, high salt concentrations, or exposure to a mildly acidic pH. SOSIP gp140 should be a useful reagent for structural and immunogenicity studies.
Figures

References
-
- Allaway, G. P., K. L. Davis-Bruno, G. A. Beaudry, E. B. Garcia, E. L. Wong, A. M. Ryder, K. W. Hasel, M. C. Gauduin, R. A. Koup, J. S. McDougal, and P. J. Maddon. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res. Hum. Retrovir. 11:533-539. - PubMed
-
- Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. - PMC - PubMed
-
- Bewley, C. A., J. M. Louis, R. Ghirlando, and G. M. Clore. 2002. Design of a novel peptide inhibitor of HIV fusion that disrupts the internal trimeric coiled-coil of gp41. J. Biol. Chem. 277:14238-14245. - PubMed
-
- Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

