Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease
- PMID: 12163609
- PMCID: PMC136419
- DOI: 10.1128/jvi.76.17.8900-8909.2002
Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease
Erratum in
- J Virol. 2003 Feb;77(4):2799
Abstract
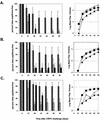
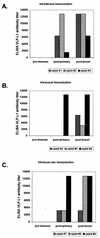
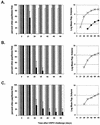
Immunizations with live recombinant vesicular stomatitis viruses (rVSV) expressing foreign viral proteins have successfully protected animals from challenges with several heterologous viruses. We developed an rVSV expressing the major capsid protein (L1) of cottontail rabbit papillomavirus (CRPV) and tested the efficacy of protection following CRPV challenge. An rVSV expressing L1 of CRPV (VSV-L1) was characterized for the protective ability afforded by intranasal, intradermal, or intramuscular vaccination in rabbits subsequently challenged with CRPV. Protein expression of L1 in VSV-L1 was confirmed by radioimmunoprecipitation assays. Nuclear localization of L1 was demonstrated by indirect immunofluorescence assays. Immunized rabbits elicited significant VSV neutralization and VLP-L1 enzyme-linked immunosorbent assay titers. VSV-L1 vaccination was not associated with weight loss or any other adverse clinical signs in the rabbit model. VSV shedding in nasal secretions occurred in some rabbits, peaking at 4 to 6 days after intranasal vaccination, with no further shedding after day 6. Specific humoral immunity to the L1 protein was consistently seen after a single VSV-L1 vaccination when administered through an intradermal or intramuscular route or after a boost via the intranasal route. Rabbits were completely protected from CRPV-induced papillomas after VSV-L1 vaccination and boost given intranasally or intramuscularly. Vaccination with VSV-L1 is a novel approach to prevent papillomavirus-induced disease and demonstrates a potential strategy for developing a human papillomavirus vaccine that can be given without injection.
Figures

Similar articles
-
Complete protection from papillomavirus challenge after a single vaccination with a vesicular stomatitis virus vector expressing high levels of L1 protein.J Virol. 2004 Mar;78(6):3196-9. doi: 10.1128/jvi.78.6.3196-3199.2004. J Virol. 2004. PMID: 14990742 Free PMC article.
-
Protective cell-mediated immunity by DNA vaccination against Papillomavirus L1 capsid protein in the Cottontail Rabbit Papillomavirus model.Viral Immunol. 2006 Summer;19(3):492-507. doi: 10.1089/vim.2006.19.492. Viral Immunol. 2006. PMID: 16987067
-
Immunisation with recombinant BCG expressing the cottontail rabbit papillomavirus (CRPV) L1 gene provides protection from CRPV challenge.Vaccine. 2006 Mar 15;24(12):2087-93. doi: 10.1016/j.vaccine.2005.11.029. Epub 2005 Nov 28. Vaccine. 2006. PMID: 16343704
-
Recombinant Listeria monocytogenes as a live vaccine vehicle and a probe for studying cell-mediated immunity.Immunol Rev. 1997 Aug;158:147-57. doi: 10.1111/j.1600-065x.1997.tb01001.x. Immunol Rev. 1997. PMID: 9314083 Review.
-
[Vesicular stomatitis virus (VSV) as a vaccine vector for immunization against viral infections].Postepy Hig Med Dosw (Online). 2013 Jan 11;67:1345-58. doi: 10.5604/17322693.1083016. Postepy Hig Med Dosw (Online). 2013. PMID: 24379275 Review. Polish.
Cited by
-
Synergistic attenuation of vesicular stomatitis virus by combination of specific G gene truncations and N gene translocations.J Virol. 2007 Feb;81(4):2056-64. doi: 10.1128/JVI.01911-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151112 Free PMC article.
-
Heterologous boosting of recombinant adenoviral prime immunization with a novel vesicular stomatitis virus-vectored tuberculosis vaccine.Mol Ther. 2008 Jun;16(6):1161-9. doi: 10.1038/mt.2008.59. Epub 2008 Mar 25. Mol Ther. 2008. PMID: 18388911 Free PMC article.
-
Single-dose, virus-vectored vaccine protection against Yersinia pestis challenge: CD4+ cells are required at the time of challenge for optimal protection.Vaccine. 2008 Nov 25;26(50):6329-37. doi: 10.1016/j.vaccine.2008.09.031. Epub 2008 Oct 1. Vaccine. 2008. PMID: 18832004 Free PMC article.
-
How will HPV vaccines affect cervical cancer?Nat Rev Cancer. 2006 Oct;6(10):753-63. doi: 10.1038/nrc1973. Nat Rev Cancer. 2006. PMID: 16990853 Free PMC article. Review.
-
A novel, live-attenuated vesicular stomatitis virus vector displaying conformationally intact, functional HIV-1 envelope trimers that elicits potent cellular and humoral responses in mice.PLoS One. 2014 Sep 12;9(9):e106597. doi: 10.1371/journal.pone.0106597. eCollection 2014. PLoS One. 2014. PMID: 25215861 Free PMC article.
References
-
- Bell, J. A., J. P. Sundberg, S. J. Ghim, J. Newsome, and A. B. Jenson. 1994. Formalin-inactivated vaccine protects against mucosal papillomavirus infection: a canine model. Pathobiology 62:194-198. - PubMed
-
- Beutner, K. R., and S. Tyring. 1997. Human papillomavirus and human disease. Am. J. Med. 102:9-15. - PubMed
-
- Brandsma, J. L. 1994. Animal models for HPV vaccine development. Papillomavirus Rep. 5:105-111. [Online.] http://www.leeds.ac.uk/lmi/pvr/pvrmain.html.
-
- Brandsma, J. L. 1994. Animal models of human papillomavirus-associated oncogenesis. Intervirology 37:189-200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

