Transition to the open state of the TolC periplasmic tunnel entrance
- PMID: 12163644
- PMCID: PMC123217
- DOI: 10.1073/pnas.162039399
Transition to the open state of the TolC periplasmic tunnel entrance
Abstract
The TolC channel-tunnel spans the bacterial outer membrane and periplasm, providing a large exit duct for protein export and multidrug efflux when recruited by substrate-engaged inner membrane complexes. The sole constriction in the single pore of the homotrimeric TolC is the periplasmic tunnel entrance, which in its resting configuration is closed by dense packing of the 12 tunnel-forming alpha-helices. Recruitment of TolC must trigger opening for substrate transit to occur, but the mechanism underlying transition from the closed to the open state is not known. The high resolution structure of TolC indicates that the tunnel helices are constrained at the entrance by a circular network of intra- and intermonomer hydrogen bonds and salt bridges. To assess how opening is achieved, we disrupted these connections and monitored changes in the aperture size by measuring the single channel conductance of TolC derivatives in black lipid bilayers. Elimination of individual connections caused incremental weakening of the circular network, accompanied by gradual relaxation from the closed state and increased flexibility of the entrance. Simultaneous abolition of the key links caused a substantial increase in conductance, generating an aperture that corresponds to the modeled open state, with the capacity to allow access and passage of diverse substrates. The results support a model in which transition to the open state of TolC is achieved by an iris-like realignment of the tunnel entrance helices.
Figures




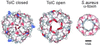
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

