Coupling complement regulators to immunoglobulin domains generates effective anti-complement reagents with extended half-life in vivo
- PMID: 12165074
- PMCID: PMC1906445
- DOI: 10.1046/j.1365-2249.2002.01924.x
Coupling complement regulators to immunoglobulin domains generates effective anti-complement reagents with extended half-life in vivo
Abstract
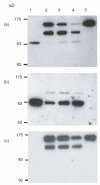
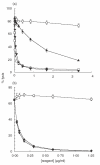
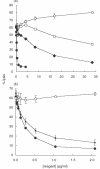
Complement activation and subsequent generation of inflammatory molecules and membrane attack complex contributes to the pathology of a number of inflammatory and degenerative diseases, including arthritis, glomerulonephritis and demyelination. Agents that specifically inhibit complement activation might prove beneficial in the treatment of these diseases. Soluble recombinant forms of the naturally occurring membrane complement regulatory proteins (CRP) have been exploited for this purpose. We have undertaken to design better therapeutics based on CRP. Here we describe the generation of soluble, recombinant CRP comprising rat decay accelerating factor (DAF) or rat CD59 expressed as Fc fusion proteins, antibody-like molecules comprising two CRP moieties in place of the antibody Fab arms (CRP-Ig). Reagents bearing DAF on each arm (DAF-Ig), CD59 on each arm (CD59-Ig) and a hybrid reagent containing both DAF and CD59 were generated. All three reagents inhibited C activation in vitro. Compared with soluble CRP lacking Fc domains, activity was reduced, but was fully restored by enzymatic release of the regulator from the Ig moiety, implicating steric constraints in reducing functional activity. In vivo studies showed that DAF-Ig, when compared to soluble DAF, had a much extended half-life in the circulation in rats and concomitantly caused a sustained reduction in plasma complement activity. When given intra-articularly to rats in a model of arthritis, DAF-Ig significantly reduced severity of disease. The data demonstrate the potential of CRP-Ig as reagents for sustained therapy of inflammatory disorders, including arthritis, but emphasize the need for careful design of fusion proteins to retain function.
Figures




References
-
- Morgan BP, Meri S. Membrane proteins that protect against complement lysis. Springer Semin Immunopathol. 1994;15:369–96. - PubMed
-
- Morgan BP, Harris CL. London: Academic Press; 1999. Complement Regulatory Proteins.
-
- Kinoshita T, Lavoie S, Nussenzweig V. Regulatory proteins for the activated third and fourth components of complement (C3b and C4b) in mice. II. Identification and properties of complement receptor type 1 (CR1) J Immunol. 1985;134:2564–70. - PubMed
-
- Reid KBM, Bentley DR, Campbell RD, Chung LP, Sim RB, Kristensen T, Tack BF. Complement proteins which interact with C3b or C4b. A superfamily of structurally related proteins. Immunol Today. 1986;7:230–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

