Assessment of thymic output in common variable immunodeficiency patients by evaluation of T cell receptor excision circles
- PMID: 12165093
- PMCID: PMC1906453
- DOI: 10.1046/j.1365-2249.2002.01893.x
Assessment of thymic output in common variable immunodeficiency patients by evaluation of T cell receptor excision circles
Abstract
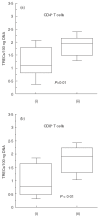
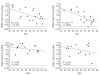
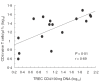
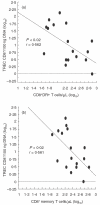
Common variable immunodeficiency (CVID) is a heterogeneous syndrome characterized by repeated infections and hypogammaglobulinaemia. Additionally, T-cell abnormalities including lymphopenia, decreased proliferation to mitogens and antigens, and the reduced production and expression of cytokines, have also been observed. In this study we have investigated the expression of naive, memory and activation markers in T-cell subpopulations in 17 CVID patients in comparison to age-matched normal controls. The numbers of CD4+ T cells, including CD45RA+CD62L+ and, to a lesser extent, CD45RA-CD62L+/RA+CD62L- were significantly reduced in patients, whereas CD8+ T cells were within normal range. In contrast, HLA-DR+ cells were increased both in CD4+ and CD8+ T cells. To assess the thymic output, we analysed the presence of T-cell receptor excision circles (TRECs) in CD4+ and CD8+ T cells by quantitative PCR. TRECs were decreased significantly in patients and the rate of TREC loss was higher with increasing age. TRECs correlated with naive CD4+ T cells, whereas there was an inverse relationship between TRECs and CD8+HLA-DR+ and CD8+CD45RA-CD62L+/RA+CD62L- T cells. Our results suggest the presence of a defect in the naive T cell compartment with origin at the thymic level in CVID, and indicate that TREC may be a useful marker to monitor thymic function in this primary immunodeficiency.
Figures
References
-
- Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunologic features of 248 patients. Clin Immunol. 1999;92:34–48. 10.1006/clim.1999.4725. - DOI - PubMed
-
- Muscaritoli M, Fanfarillo F, Luzi G, et al. Impaired nutritional status in CVID patients correlates with reduced levels of serum IgA and of circulating CD4 T lymphocytes. Eur J Clin Invest. 2001;31:544–9. - PubMed
-
- Hammarström L, Smith CIE. Genetic approach to common variable immunodeficiency and IgA deficiency. In: Ochs HD, Smith CIE, Puck JM, editors. Primary immunodeficiency diseases. New York: Oxford University Press; 1999. pp. 250–62.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

