Combination of molecular mimicry and aberrant autoantigen expression is important for development of anti-Fas ligand autoantibodies in patients with systemic lupus erythematosus
- PMID: 12165095
- PMCID: PMC1906439
- DOI: 10.1046/j.1365-2249.2002.01812.x
Combination of molecular mimicry and aberrant autoantigen expression is important for development of anti-Fas ligand autoantibodies in patients with systemic lupus erythematosus
Abstract
We have reported previously that circulating anti-Fas ligand (FasL) autoantibodies able to inhibit Fas/FasL-mediated apoptosis were present in patients with systemic lupus erythematosus (SLE). In the present study, we describe the epitopes recognized by these anti-FasL autoantibodies. Rabbit antihuman antibody, raised against a FasL fragment consisting of amino acids (aa) 103-179 (fragment 2.0), inhibited Fas/FasL-mediated apoptosis, whereas an antibody against a FasL aa 103-146 fragment (fragment 1.0) did not. This suggested that an epitope around aa 146-179 was important for Fas/FasL interaction. Epitope mapping of anti-FasL autoantibodies using deletion mutants indicated that the epitope was located around aa 163-179. Three-dimensional molecular modelling of the Fas/FasL complex revealed that the aa 162-169 region was located on the outermost side of FasL, which suggested that the anti-FasL autoantibody would easily have access to the epitope. FasL point mutants involving aa positions 162-169 resulted in complete loss of apoptosis-inducing capability, which suggested that the aa 162-169 region was important for Fas/FasL interaction. A synthetic FasL peptide consisting of aa 161-170 blocked the binding of anti-FasL autoantibodies to FasL fragment 2.0 (aa 103-179). The FasL aa 161-170 sequence was found to be highly homologous with aa sequences from several infectious agents. Synthetic peptides derived from some of these microorganisms cross-reacted with the epitope recognized by the autoantibodies, suggesting that several foreign infectious agent-derived proteins may share an epitope with human FasL. As lymphocytes from SLE patients aberrartly expressed FasL, it is possible that infection by one of several infectious agents may trigger cross-reactive antibody responses, after which aberrantly expressed endogenous FasL might induce the shift from a cross-reactive response to an authentic autoimmune response. Therefore, a combination of molecular mimicry and aberrant autoantigen expression may be important for the development of anti-FasL autoantibodies in SLE patients.
Figures
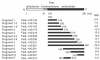
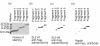
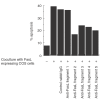


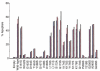
 , 0·8 ng/ml;
, 0·8 ng/ml;  , 1·6 ng/ml;
, 1·6 ng/ml;  , 3·2 ng/ml.
, 3·2 ng/ml.

References
-
- Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–68. - PubMed
-
- Madaio MP. B cells and autoantibodies in the pathogenesis of lupus nephritis. Immunol Res. 1998;17:123–32. - PubMed
-
- Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–6. - PubMed
-
- Radic MZ, Weigert M. Origins of anti-DNA antibodies and their implications for B-cell tolerance. Ann NY Acad Sci. 1995;764:384–96. - PubMed
-
- Canbral AR, Alarcon-Segovia D. Autoantibodies in systemic lupus erythematosus. Curr Opin Rheumatol. 1998;10:409–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

