Amygdala-hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data
- PMID: 12165365
- PMCID: PMC2824647
- DOI: 10.1016/s0925-4927(02)00025-2
Amygdala-hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data
Abstract
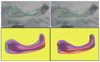
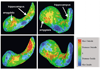
Evidence suggests that some structural brain abnormalities in schizophrenia are neurodevelopmental in origin. There is also growing evidence to suggest that shape deformations in brain structure may reflect abnormalities in neurodevelopment. While many magnetic resonance (MR) imaging studies have investigated brain area and volume measures in schizophrenia, fewer have focused on shape deformations. In this MR study we used a 3D shape representation technique, based on spherical harmonic functions, to analyze left and right amygdala-hippocampus shapes in each of 15 patients with schizophrenia and 15 healthy controls matched for age, gender, handedness and parental socioeconomic status. Left/right asymmetry was also measured for both shape and volume differences. Additionally, shape and volume measurements were combined in a composite analysis. There were no differences between groups in overall volume or shape. Left/right amygdala-hippocampal asymmetry, however, was significantly larger in patients than controls for both relative volume and shape. The local brain regions responsible for the left/right asymmetry differences in patients with schizophrenia were in the tail of the hippocampus (including both the inferior aspect adjacent to parahippocampal gyrus and the superior aspect adjacent to the lateral geniculate nucleus and more anteriorly to the cerebral peduncles) and in portions of the amygdala body (including the anterior-superior aspect adjacent to the basal nucleus). Also, in patients, increased volumetric asymmetry tended to be correlated with increased left/right shape asymmetry. Furthermore, a combined analysis of volume and shape asymmetry resulted in improved differentiation between groups. Classification function analyses correctly classified 70% of cases using volume, 73.3% using shape, and 87% using combined volume and shape measures. These findings suggest that shape provides important new information toward characterizing the pathophysiology of schizophrenia, and that combining volume and shape measures provides improved group discrimination in studies investigating brain abnormalities in schizophrenia. An evaluation of shape deformations also suggests local abnormalities in the amygdala-hippocampal complex in schizophrenia.
Figures


References
-
- American Psychiatric Association on Nomenclature and Statistics. Diagnostic and Statistical Manual of Mental Disorders, revised. 3rd edition. Washington, DC: American Psychiatric Association; 1987.
-
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa College of Medicine; 1981.
-
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa College of Medicine; 1984.
-
- Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V, II, O’Leary DS, Ehrhardt JC, Yuh WTC. Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. JAMA. 1994;272:1763–1769. - PubMed
-
- Angenent S, Haker S, Tannenbaum A, Kikinis R. On the Laplace–Beltrami operator and brain surface flattening. IEEE Transactions on Medical Imaging. 1999;18:700–711. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

