Interactions between lipids and bacterial reaction centers determined by protein crystallography
- PMID: 12167672
- PMCID: PMC123209
- DOI: 10.1073/pnas.162368399
Interactions between lipids and bacterial reaction centers determined by protein crystallography
Abstract
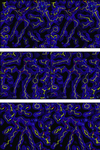
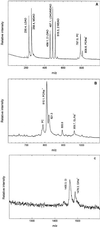
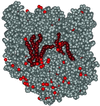
The structure of the reaction center from Rhodobacter sphaeroides has been solved by using x-ray diffraction at a 2.55-A resolution limit. Three lipid molecules that lie on the surface of the protein are resolved in the electron density maps. In addition to a cardiolipin that has previously been reported [McAuley, K. E., Fyfe, P. K., Ridge, J. P., Isaacs, N. W., Cogdell, R. J. & Jones, M. R. (1999) Proc. Natl. Acad. Sci. USA 96, 14706-14711], two other major lipids of the cell membrane are found, a phosphatidylcholine and a glucosylgalactosyl diacylglycerol. The presence of these three lipids has been confirmed by laser mass spectroscopy. The lipids are located in the hydrophobic region of the protein surface and interact predominately with hydrophobic amino acids, in particular aromatic residues. Although the cardiolipin is over 15 A from the cofactors, the other two lipids are in close contact with the cofactors and may contribute to the difference in energetics for the two branches of cofactors that is primarily responsible for the asymmetry of electron transfer. The glycolipid is 3.5 A from the active bacteriochlorophyll monomer and shields this cofactor from the solvent in contrast to a much greater exposed surface evident for the inactive bacteriochlorophyll monomer. The phosphate atom of phosphatidylcholine is 6.5 A from the inactive bacteriopheophytin, and the associated electrostatic interactions may contribute to electron transfer rates involving this cofactor. Overall, the lipids span a distance of approximately 30 A, which is consistent with a bilayer-like arrangement suggesting the presence of an "inner shell" of lipids around membrane proteins that is critical for membrane function.
Figures

References
-
- Benning C. (1998) in Lipids in Photosynthesis: Structure, Function, and Genetics, eds. Siegenthaler, P. A. & Murata, N. (Kluwer, Dordrecht, The Netherlands), pp. 83–101.
-
- Michel H., (1991) Crystallization of Membrane Proteins (CRC, Boca Raton, FL).
-
- Feher G., Allen, J. P., Okamura, M. Y. & Rees, D. C. (1989) Nature (London) 339, 111-116.
-
- Blankenship R. E., Madigan, M. T. & Bauer, C. E., (1995) Anoxygenic Photosynthetic Bacteria (Kluwer, Dordrecht, The Netherlands).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

