Analysis and classification of cerebellar malformations
- PMID: 12169461
- PMCID: PMC8185716
Analysis and classification of cerebellar malformations
Abstract
Background and purpose: Because of improved visualization of posterior fossa structures with MR imaging, cerebellar malformations are recognized with increasing frequency. Herein we attempt to describe and propose a rational classification of cerebellar malformations.
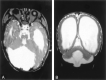
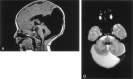
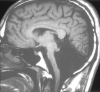
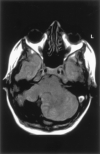
Methods: MR images obtained in 70 patients with cerebellar malformations were retrospectively reviewed. The cerebellar malformations were initially divided into those with hypoplasia and those with dysplasia. They were then divided into focal and diffuse malformations. Finally, they were separated according to other features, such as brain stem involvement and cerebral involvement.
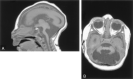
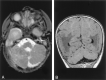
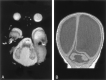
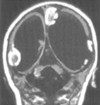
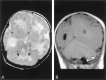
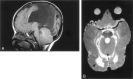
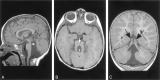
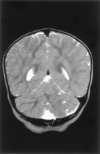
Results: All patients with diffuse cerebellar dysplasia (muscular dystrophy [n = 10], cytomegalovirus [n = 6], lissencephaly [n = 3],) had abnormalities of the cerebrum. Patients with focal cerebellar dysplasia of the Joubert (n = 12) and rhombencephalosynapsis (n = 8) types had variable cerebral dysplasia. Patients with nonsyndromic focal cerebellar dysplasia (isolated focal cerebellar cortical dysplasia [n = 2], cerebellar heterotopia with cerebellar cortical dysplasia [n = 1], idiopathic diffuse cerebellar dysplasia [n = 1], Lhermitte-Duclos syndrome [n = 1]) and those with cerebellar hypoplasia (isolated cerebellar hypoplasia [n = 6], pontocerebellar hypoplasia type 1 [n = 1]) had normal cerebra. Patients with features of Dandy-Walker malformation (n = 19) had both hypoplasia and dysplasia of the cerebellum. No notable difference was found between the cerebella of patients with large fourth ventricle cysts (Dandy-Walker malformations) and those without large fourth ventricle cysts (isolated cerebellar hypoplasia). Therefore, the Dandy-Walker malformation seems to be heterogeneous.
Conclusion: Use of this classification system helps in the segregation and understanding of the relationship among cerebellar malformations. Although it will undoubtedly require revisions, this classification is a first step in combining imaging with molecular biology to facilitate understanding of cerebellar development and maldevelopment.
Figures
Comment in
-
Analysis and classification of cerebellar malformations.AJNR Am J Neuroradiol. 2003 Jan;24(1):153; author reply 153. AJNR Am J Neuroradiol. 2003. PMID: 12533349 Free PMC article. No abstract available.
References
-
- Raybaud C. Cystic malformations of the posterior fossa: abnormalities associated with development of the roof of the fourth ventricle and adjacent meningeal structures. J Neuroradiol 1982;9:103–133 - PubMed
-
- Tortori-Donati P, Fondelli M, Rossi A, Carini S. Cystic malformations of the posterior carnial fossa originating from a defect of the posterior membranous area: mega cisternl magna and persisting Blake’s pouch: two separate entities. Childs Nerv Syst 1996;12:303–308 - PubMed
-
- Calabrò F, Arcuri T, Jinkins JR. Blake’s pouch cyst: an entity within the Dandy-Walker continuum. Neuroradiology 2000;42:290–295 - PubMed
-
- Barkovich AJ, Kjos BO, Norman D, Edwards MS. Revised classification of posterior fossa cysts and cyst-like malformations based on results of multiplanar MR imaging. AJNR Am J Neuroradiol 1989;10:977–988 - PubMed
-
- Satran D, Pierpont ME, Dobyns WB. Cerebello-Oculo-Renal syndromes including Arima, Senior-Löken and COACH syndromes: more than just variants of Joubert syndrome. Am J Med Genet 1999;86:459–469 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
