Tet(L) and tet(K) tetracycline-divalent metal/H+ antiporters: characterization of multiple catalytic modes and a mutagenesis approach to differences in their efflux substrate and coupling ion preferences
- PMID: 12169596
- PMCID: PMC135290
- DOI: 10.1128/JB.184.17.4722-4732.2002
Tet(L) and tet(K) tetracycline-divalent metal/H+ antiporters: characterization of multiple catalytic modes and a mutagenesis approach to differences in their efflux substrate and coupling ion preferences
Abstract
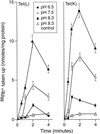
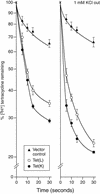
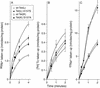
The Tet(L) protein encoded in the Bacillus subtilis chromosome and the closely related Tet(K) protein from Staphylococcus aureus plasmids are multifunctional antiporters that have three cytoplasmic efflux substrates: a tetracycline-divalent metal (TC-Me(2+)) complex that bears a net single positive charge, Na+, and K+. Tet(L) and Tet(K) had been shown to couple efflux of each of these substrates to influx of H+ as the coupling ion. In this study, competitive cross-inhibition between K+ and other cytoplasmic efflux substrates was demonstrated. Tet(L) and Tet(K) had also been shown to use K+ as an alternate coupling ion in support of Na+ or K+ efflux. Here they were shown to couple TC-Me(2+) efflux to K+ uptake as well, exhibiting greater use of K+ as a coupling ion as the external pH increased. The substrate and coupling ion preferences of the two Tet proteins differed, especially in the higher preference of Tet(K) than Tet(L) for K+, both as a cytoplasmic efflux substrate and as an external coupling ion. Site-directed mutagenesis was employed to test the hypothesis that some feature of the putative "antiporter motif," motif C, of Tet proteins would be involved in these characteristic preferences. Mutation of the A157 in Tet(L) to a hydroxyamino acid resulted in a more Tet(K)-like K+ preference both as coupling ion and efflux substrate. A reciprocal S157A mutant of Tet(K) exhibited reduced K+ preference. Competitive inhibition among substrates and the parallel effects of the single mutation upon K+ preference, as both an efflux substrate and coupling ion, are compatible with a model in which a single translocation pathway through the Tet(L) and Tet(K) transporters is used both for the cytoplasmic efflux substrates and for the coupling ions, in an alternating fashion. However, the effects of the A157 and other mutations of Tet(L) indicate that even if there are a shared binding site and translocation pathway, some elements of that pathway are used by all substrates and others are important only for particular substrates.
Figures



References
-
- Blostein, R., A. Wilczynska, S. J. D. Karlish, J. M. Arguello, and J. B. Lingrel. 1999. Evidence that Ser775 in the alpha subunit of the Na,K-ATPase is a residue in the cation binding pocket. J. Biol. Chem. 272:24987-24993. - PubMed
-
- Cheng, J., A. A. Guffanti, and T. A. Krulwich. 1994. The chromosomal tetracycline resistance locus of Bacillus subtilis encodes a Na+/H+ antiporter that is physiologically important at elevated pH. J. Biol. Chem. 269:27365-27371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

