Maternally transmitted severe glucose 6-phosphate dehydrogenase deficiency is an embryonic lethal
- PMID: 12169625
- PMCID: PMC126165
- DOI: 10.1093/emboj/cdf426
Maternally transmitted severe glucose 6-phosphate dehydrogenase deficiency is an embryonic lethal
Abstract
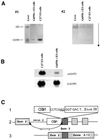
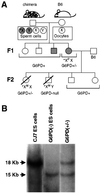
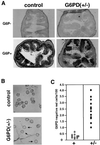
Mouse chimeras from embryonic stem cells in which the X-linked glucose 6-phosphate dehydrogenase (G6PD) gene had been targeted were crossed with normal females. First-generation (F(1)) G6PD(+/-) heterozygotes born from this cross were essentially normal; analysis of their tissues demonstrated strong selection for cells with the targeted G6PD allele on the inactive X chromosome. When these F(1) G6PD(+/-) females were bred to normal males, only normal G6PD mice were born, because: (i) hemizygous G6PD(-) male embryos died by E10.5 and their development was arrested from E7.5, the time of onset of blood circulation; (ii) heterozygous G6PD(+/-) females showed abnormalities from E8.5, and died by E11.5; and (iii) severe pathological changes were present in the placenta of both G6PD(-) and G6PD(+/-) embryos. Thus, G6PD is not indispensable for early embryo development; however, severe G6PD deficiency in the extraembryonic tissues (consequent on selective inactivation of the normal paternal G6PD allele) impairs the development of the placenta and causes death of the embryo. Most importantly, G6PD is indispensable for survival when the embryo is exposed to oxygen through its blood supply.
Figures
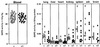




References
-
- Beutler E. (1978) Glucose 6-phosphate dehydrogenase deficiency. In Beutler,E. (ed.), Hemolytic Anemia in Disorders of Red Cell Metabolism. Plenum Medical Book Co., New York, NY, pp. 23–167.
-
- Beutler E. (1991) Glucose 6-phosphate dehydrogenase deficiency. N. Engl. J. Med., 324, 169–174. - PubMed
-
- Beutler E. (1995) Glucose 6-Phosphate Dehydrogenase Deficiency. McGraw-Hill, New York, NY.
-
- Beutler E. and Vulliamy,T. (2002) Hematologically important mutations: glucose 6-phosphate dehydrogenase. Blood Cells Mol. Dis., 28, 93–103. - PubMed
-
- Campbell F., Fuller,C.E., Williams,G.T. and Williams,E.D. (1994) Human colonic stem cell mutation frequency with and without irradiation. J. Pathol., 174, 175–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

