Mutations in the linker domain of NBD2 of SUR inhibit transduction but not nucleotide binding
- PMID: 12169627
- PMCID: PMC125404
- DOI: 10.1093/emboj/cdf419
Mutations in the linker domain of NBD2 of SUR inhibit transduction but not nucleotide binding
Abstract
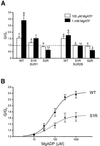
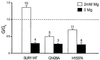
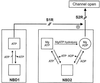
ATP-sensitive potassium (K(ATP)) channels are composed of an ATP-binding cassette (ABC) protein (SUR1, SUR2A or SUR2B) and an inwardly rectifying K(+) channel (Kir6.1 or Kir6.2). Like other ABC proteins, the nucleotide binding domains (NBDs) of SUR contain a highly conserved "signature sequence" (the linker, LSGGQ) whose function is unclear. Mutation of the conserved serine to arginine in the linker of NBD1 (S1R) or NBD2 (S2R) did not alter the ability of ATP or ADP (100 microM) to displace 8-azido-[(32)P]ATP binding to SUR1, or abolish ATP hydrolysis at NBD2. We co-expressed Kir6.2 with wild-type or mutant SUR in Xenopus oocytes and recorded the resulting currents in inside-out macropatches. The S1R mutation in SUR1, SUR2A or SUR2B reduced K(ATP) current activation by 100 microM MgADP, whereas the S2R mutation in SUR1 or SUR2B (but not SUR2A) abolished MgADP activation completely. The linker mutations also reduced (S1R) or abolished (S2R) MgATP-dependent activation of Kir6.2-R50G co-expressed with SUR1 or SUR2B. These results suggest that the linker serines are not required for nucleotide binding but may be involved in transducing nucleotide binding into channel activation.
Figures




Similar articles
-
The Kir6.2-F333I mutation differentially modulates KATP channels composed of SUR1 or SUR2 subunits.J Physiol. 2007 Jun 15;581(Pt 3):1259-69. doi: 10.1113/jphysiol.2007.130211. Epub 2007 Mar 29. J Physiol. 2007. PMID: 17395632 Free PMC article.
-
A functional role of the C-terminal 42 amino acids of SUR2A and SUR2B in the physiology and pharmacology of cardiovascular ATP-sensitive K(+) channels.J Mol Cell Cardiol. 2005 Jul;39(1):1-6. doi: 10.1016/j.yjmcc.2004.11.022. Epub 2005 Feb 5. J Mol Cell Cardiol. 2005. PMID: 15978900
-
Activation of the K(ATP) channel by Mg-nucleotide interaction with SUR1.J Gen Physiol. 2010 Oct;136(4):389-405. doi: 10.1085/jgp.201010475. J Gen Physiol. 2010. PMID: 20876358 Free PMC article.
-
[Activation of ATP-sensitive K+ channels by ADP and K+ channel openers: homology model of sulfonylurea receptor carboxyl-termini].Nihon Yakurigaku Zasshi. 2001 Sep;118(3):177-86. doi: 10.1254/fpj.118.177. Nihon Yakurigaku Zasshi. 2001. PMID: 11577458 Review. Japanese.
-
K(ATP) channel action in vascular tone regulation: from genetics to diseases.Sheng Li Xue Bao. 2012 Feb 25;64(1):1-13. Sheng Li Xue Bao. 2012. PMID: 22348955 Free PMC article. Review.
Cited by
-
Sulphonylurea action revisited: the post-cloning era.Diabetologia. 2003 Jul;46(7):875-91. doi: 10.1007/s00125-003-1143-3. Epub 2003 Jun 18. Diabetologia. 2003. PMID: 12819907 Review.
-
The unusual stoichiometry of ADP activation of the KATP channel.Front Physiol. 2014 Jan 28;5:11. doi: 10.3389/fphys.2014.00011. eCollection 2014. Front Physiol. 2014. PMID: 24478723 Free PMC article.
-
From glucose sensing to exocytosis: takes from maturity onset diabetes of the young.Front Endocrinol (Lausanne). 2023 May 15;14:1188301. doi: 10.3389/fendo.2023.1188301. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37255971 Free PMC article. Review.
-
Role for SUR2A ED domain in allosteric coupling within the K(ATP) channel complex.J Gen Physiol. 2008 Mar;131(3):185-96. doi: 10.1085/jgp.200709852. J Gen Physiol. 2008. PMID: 18299394 Free PMC article.
-
Potassium channel regulation.EMBO Rep. 2003 Nov;4(11):1038-42. doi: 10.1038/sj.embor.embor7400003. EMBO Rep. 2003. PMID: 14593442 Free PMC article. Review.
References
-
- Aguilar-Bryan L., Nichols,C.G., Wechsler,S.W., Clement,J.P.,IV, Boyd,A.E.,III, Gonzalez,G., Herrera-Sosa,H., Nguy,K., Bryan,J. and Nelson,D.A. (1995) Cloning of the β-cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science, 268, 423–426. - PubMed
-
- Aguilar-Bryan L. and Bryan,J. (1999) Molecular biology of adenosine triphosphate-sensitve potassium channels. Endocr. Rev., 20, 101–135. - PubMed
-
- Aleksandrov L., Mengos,A., Chang,X., Aleksandrov,A. and Riordan,J.R. (2001) Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem., 276, 12918–12923. - PubMed
-
- Ashcroft F.M. and Gribble,F.M. (1998) Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci., 21, 288–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

