A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands
- PMID: 12169630
- PMCID: PMC125406
- DOI: 10.1093/emboj/cdf434
A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands
Abstract
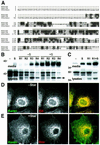
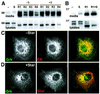
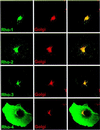
Drosophila has three membrane-tethered epidermal growth factor (EGF)-like proteins: Spitz, Gurken and Keren. Spitz and Gurken have been genetically confirmed to activate the EGF receptor, but Keren is uncharacterized. Spitz is activated by regulated intracellular translocation and cleavage by the transmembrane proteins Star and the protease Rhomboid-1, respectively. Rhomboid-1 is a member of a family of seven similar proteins in Drosophila. We have analysed four of these: all are proteases that can cleave Spitz, Gurken and Keren, and all activate only EGF receptor signalling in vivo. Star acts as an endoplasmic reticulum (ER) export factor for all three. The importance of this translocation is highlighted by the fact that when Spitz is cleaved by Rhomboids in the ER it cannot be secreted. Keren activates the EGF receptor in vivo, providing strong evidence that it is a true ligand. Our data demonstrate that all membrane-tethered EGF ligands in Drosophila are activated by the same strategy of cleavage by Rhomboids, which are ancient and widespread intramembrane proteases. This is distinct from the metalloprotease-induced activation of mammalian EGF-like ligands.
Figures




Similar articles
-
Conservation of intramembrane proteolytic activity and substrate specificity in prokaryotic and eukaryotic rhomboids.Curr Biol. 2002 Sep 3;12(17):1507-12. doi: 10.1016/s0960-9822(02)01092-8. Curr Biol. 2002. PMID: 12225666
-
Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila.Cell. 2001 Oct 19;107(2):161-71. doi: 10.1016/s0092-8674(01)00526-8. Cell. 2001. PMID: 11672524
-
Expression in mammalian cell cultures reveals interdependent, but distinct, functions for Star and Rhomboid proteins in the processing of the Drosophila transforming-growth-factor-alpha homologue Spitz.Biochem J. 2002 Apr 15;363(Pt 2):347-52. doi: 10.1042/0264-6021:3630347. Biochem J. 2002. PMID: 11931664 Free PMC article.
-
EGF receptor signalling: the importance of presentation.Curr Biol. 2000 May 18;10(10):R388-91. doi: 10.1016/s0960-9822(00)00485-1. Curr Biol. 2000. PMID: 10837218 Review.
-
Emerging role of rhomboid family proteins in mammalian biology and disease.Biochim Biophys Acta. 2013 Dec;1828(12):2840-8. doi: 10.1016/j.bbamem.2013.03.025. Epub 2013 Apr 3. Biochim Biophys Acta. 2013. PMID: 23562403 Review.
Cited by
-
The crystal structure of the rhomboid peptidase from Haemophilus influenzae provides insight into intramembrane proteolysis.Proc Natl Acad Sci U S A. 2007 Jan 16;104(3):750-4. doi: 10.1073/pnas.0609981104. Epub 2007 Jan 8. Proc Natl Acad Sci U S A. 2007. PMID: 17210913 Free PMC article.
-
Nicastrin functions to sterically hinder γ-secretase-substrate interactions driven by substrate transmembrane domain.Proc Natl Acad Sci U S A. 2016 Feb 2;113(5):E509-18. doi: 10.1073/pnas.1512952113. Epub 2015 Dec 22. Proc Natl Acad Sci U S A. 2016. PMID: 26699478 Free PMC article.
-
Glia relay differentiation cues to coordinate neuronal development in Drosophila.Science. 2017 Sep 1;357(6354):886-891. doi: 10.1126/science.aan3174. Science. 2017. PMID: 28860380 Free PMC article.
-
The Role of iRhom2 in Metabolic and Cardiovascular-Related Disorders.Front Cardiovasc Med. 2020 Nov 24;7:612808. doi: 10.3389/fcvm.2020.612808. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 33330676 Free PMC article. Review.
-
The rhomboid protease family: a decade of progress on function and mechanism.Genome Biol. 2011 Oct 27;12(10):231. doi: 10.1186/gb-2011-12-10-231. Genome Biol. 2011. PMID: 22035660 Free PMC article. Review.
References
-
- Adamson E.D. (1990) Developmental activities of the epidermal growth factor receptor. Curr. Top. Dev. Biol., 24, 1–29. - PubMed
-
- Arribas J., Coodly,L., Vollmer,P., Kishimoto,T.K., Rose-John,S. and Massagué,J. (1996) Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J. Biol. Chem., 271, 11376–11382. - PubMed
-
- Baker N.E. and Yu,S.Y. (2001) The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell, 104, 699–708. - PubMed
-
- Baonza A., Casci,T. and Freeman,M. (2001) A primary role for the EGF receptor in ommatidial spacing in the Drosophila eye. Curr. Biol., 11, 396–404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

