A 5'-3' long-range interaction in Ty1 RNA controls its reverse transcription and retrotransposition
- PMID: 12169639
- PMCID: PMC126173
- DOI: 10.1093/emboj/cdf436
A 5'-3' long-range interaction in Ty1 RNA controls its reverse transcription and retrotransposition
Abstract
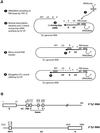
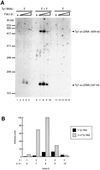
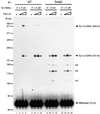
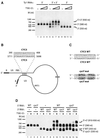
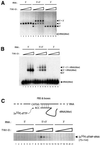
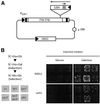
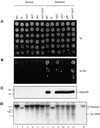
LTR-retrotransposons are abundant components of all eukaryotic genomes and appear to be key players in their evolution. They share with retroviruses a reverse transcription step during their replication cycle. To better understand the replication of retrotransposons as well as their similarities to and differences from retroviruses, we set up an in vitro model system to examine minus-strand cDNA synthesis of the yeast Ty1 LTR-retrotransposon. Results show that the 5' and 3' ends of Ty1 genomic RNA interact through 14 nucleotide 5'-3' complementary sequences (CYC sequences). This 5'-3' base pairing results in an efficient initiation of reverse transcription in vitro. Transposition of a marked Ty1 element and Ty1 cDNA synthesis in yeast rely on the ability of the CYC sequences to base pair. This 5'-3' interaction is also supported by phylogenic analysis of all full-length Ty1 and Ty2 elements present in the Saccharomyces cerevisiae genome. These novel findings lead us to propose that circularization of the Ty1 genomic RNA controls initiation of reverse transcription and may limit reverse transcription of defective retroelements.
Figures



References
-
- Adams S.E., Mellor,J., Gull,K., Sim,R.B., Tuite,M.F., Kingsman,S.M. and Kingsman,A.J. (1987) The functions and relationships of Ty-VLP proteins in yeast reflect those of mammalian retroviral proteins. Cell, 49, 111–119. - PubMed
-
- Ausubel F.M. (1995) Short Protocols in Molecular Biology: A Compendium of Methods from Current Protocols in Molecular Biology. Wiley, New York, NY.
-
- Berkhout B., Ooms,M., Beerens,N., Huthoff,H., Southern,E. and Verhoef,K. (2002) In vitro evidence that the untranslated leader of the HIV-1 genome is an RNA checkpoint that regulates multiple functions through conformational changes. J. Biol. Chem., 277, 19967–19975. - PubMed
-
- Boeke J.D. and Stoye,J.P. (1997) Retrotransposons, endogenous retroviruses and the evolution of retroelements. In Cofin,J.M., Hughes,S.H. and Varmus,H.E. (eds), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 343–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

