Tyrosine sulfation of CCR5 N-terminal peptide by tyrosylprotein sulfotransferases 1 and 2 follows a discrete pattern and temporal sequence
- PMID: 12169668
- PMCID: PMC123205
- DOI: 10.1073/pnas.172380899
Tyrosine sulfation of CCR5 N-terminal peptide by tyrosylprotein sulfotransferases 1 and 2 follows a discrete pattern and temporal sequence
Abstract
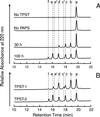
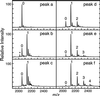
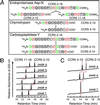
The CC-chemokine receptor 5 (CCR5) is the major coreceptor for the entry of macrophage-tropic (R5) HIV-1 strains into target cells. Posttranslational sulfation of tyrosine residues in the N-terminal tail of CCR5 is critical for high affinity interaction of the receptor with the HIV-1 envelope glycoprotein gp120 in complex with CD4. Here, we focused on defining precisely the sulfation pattern of the N terminus of CCR5 by using recombinant human tyrosylprotein sulfotransferases TPST-1 and TPST-2 to modify a synthetic peptide that corresponds to amino acids 2-18 of the receptor (CCR5 2-18). Analysis of the reaction products was made with a combination of reversed-phase HPLC, proteolytic cleavage, and matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS). We found that CCR5 2-18 is sulfated by both TPST isoenzymes leading to a final product with four sulfotyrosine residues. Sulfates were added stepwise to the peptide producing specific intermediates with one, two, or three sulfotyrosines. The pattern of sulfation in these intermediates suggests that Tyr-14 and Tyr-15 are sulfated first, followed by Tyr-10, and finally Tyr-3. These results represent a detailed analysis of the multiple sulfation reaction of a peptide substrate by TPSTs and provide a structural basis for understanding the role of tyrosine sulfation of CCR5 in HIV-1 coreceptor and chemokine receptor function.
Figures

Similar articles
-
Sequential tyrosine sulfation of CXCR4 by tyrosylprotein sulfotransferases.Biochemistry. 2008 Oct 28;47(43):11251-62. doi: 10.1021/bi800965m. Epub 2008 Oct 4. Biochemistry. 2008. PMID: 18834145 Free PMC article.
-
Pattern and temporal sequence of sulfation of CCR5 N-terminal peptides by tyrosylprotein sulfotransferase-2: an assessment of the effects of N-terminal residues.Biochemistry. 2009 Jun 16;48(23):5332-8. doi: 10.1021/bi900285c. Biochemistry. 2009. PMID: 19402700 Free PMC article.
-
Preparation and Analysis of N-Terminal Chemokine Receptor Sulfopeptides Using Tyrosylprotein Sulfotransferase Enzymes.Methods Enzymol. 2016;570:357-88. doi: 10.1016/bs.mie.2015.09.004. Epub 2015 Nov 14. Methods Enzymol. 2016. PMID: 26921955 Free PMC article.
-
The Synthesis of Sulfated CCR5 Peptide Surrogates and their Use to Study Receptor-Ligand Interactions.Protein Pept Lett. 2018;25(12):1124-1136. doi: 10.2174/0929866525666181101103834. Protein Pept Lett. 2018. PMID: 30381052 Review.
-
Determinants of tyrosylprotein sulfation coding and substrate specificity of tyrosylprotein sulfotransferases in metazoans.Chem Biol Interact. 2016 Nov 25;259(Pt A):17-22. doi: 10.1016/j.cbi.2016.04.006. Epub 2016 Apr 7. Chem Biol Interact. 2016. PMID: 27062897 Review.
Cited by
-
Preparation of Tyrosylprotein Sulfotransferases for In Vitro One-Pot Enzymatic Synthesis of Sulfated Proteins/Peptides.ACS Omega. 2018 Sep 30;3(9):11633-11642. doi: 10.1021/acsomega.7b01533. Epub 2018 Sep 24. ACS Omega. 2018. PMID: 30320268 Free PMC article.
-
Direct identification of tyrosine sulfation by using ultraviolet photodissociation mass spectrometry.J Am Soc Mass Spectrom. 2014 Aug;25(8):1461-71. doi: 10.1007/s13361-014-0910-3. Epub 2014 May 21. J Am Soc Mass Spectrom. 2014. PMID: 24845354 Free PMC article.
-
Chemokine receptor type-5: a key regulator of immunity, disease pathogenesis, and emerging therapeutic target.Inflammopharmacology. 2025 Aug;33(8):4477-4498. doi: 10.1007/s10787-025-01871-2. Epub 2025 Aug 8. Inflammopharmacology. 2025. PMID: 40779011 Review.
-
Structural basis for chemokine recognition and receptor activation of chemokine receptor CCR5.Nat Commun. 2021 Jul 6;12(1):4151. doi: 10.1038/s41467-021-24438-5. Nat Commun. 2021. PMID: 34230484 Free PMC article.
-
A potential antibody repertoire diversification mechanism through tyrosine sulfation for biotherapeutics engineering and production.Front Immunol. 2022 Dec 8;13:1072702. doi: 10.3389/fimmu.2022.1072702. eCollection 2022. Front Immunol. 2022. PMID: 36569848 Free PMC article.
References
-
- Combadiere C., Ahuja, S. K., Tiffany, H. L. & Murphy, P. M. (1996) J. Leukocyte Biol. 60, 147-152. - PubMed
-
- Samson M., Labbe, O., Mollereau, C., Vassart, G. & Parmentier, M. (1996) Biochemistry 35, 3362-3367. - PubMed
-
- Raport C. J., Gosling, J., Schweickart, V. L., Gray, P. W. & Charo, I. F. (1996) J. Biol. Chem. 271, 17161-17166. - PubMed
-
- Gong W., Howard, O. M., Turpin, J. A., Grimm, M. C., Ueda, H., Gray, P. W., Raport, C. J., Oppenheim, J. J. & Wang, J. M. (1998) J. Biol. Chem. 273, 4289-4292. - PubMed
-
- Murphy P. M., Baggiolini, M., Charo, I. F., Hebert, C. A., Horuk, R., Matsushima, K., Miller, L. H., Oppenheim, J. J. & Power, C. A. (2000) Pharmacol. Rev. 52, 145-176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

