A nuclear export signal within the high mobility group domain regulates the nucleocytoplasmic translocation of SOX9 during sexual determination
- PMID: 12169669
- PMCID: PMC123233
- DOI: 10.1073/pnas.172383099
A nuclear export signal within the high mobility group domain regulates the nucleocytoplasmic translocation of SOX9 during sexual determination
Abstract
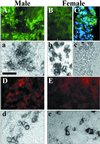
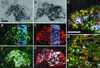
In mammals, male sex determination starts when the Y chromosome Sry gene is expressed within the undetermined male gonad. One of the earliest effect of Sry expression is to induce up-regulation of Sox9 gene expression in the developing gonad. SOX9, like SRY, contains a high mobility group domain and is sufficient to induce testis differentiation in transgenic XX mice. Before sexual differentiation, SOX9 protein is initially found in the cytoplasm of undifferentiated gonads from both sexes. At the time of testis differentiation and anti-Müllerian hormone expression, it becomes localized to the nuclear compartment in males whereas it is down-regulated in females. In this report, we used NIH 3T3 cells as a model to examine the regulation of SOX9 nucleo-cytoplasmic shuttling. SOX9-transfected cells expressed nuclear and cytoplasmic SOX9 whereas transfected cells treated with the nuclear export inhibitor leptomycin B, displayed an exclusive nuclear localization of SOX9. By using SOX9 deletion constructs in green fluorescent protein fusion proteins, we identified a functional nuclear export signal sequence between amino acids 134 and 147 of SOX9 high mobility group box. More strikingly, we show that inhibiting nuclear export with leptomycin B in mouse XX gonads cultured in vitro induced a sex reversal phenotype characterized by nuclear SOX9 and anti-Müllerian hormone expression. These results indicate that SOX9 nuclear export signal is essential for SOX9 sex-specific subcellular localization and could be part of a regulatory switch repressing (in females) or triggering (in males) male-specific sexual differentiation.
Figures




Similar articles
-
Sry and Sox9 expression during canine gonadal sex determination assayed by quantitative reverse transcription-polymerase chain reaction.Mol Reprod Dev. 2003 Aug;65(4):373-81. doi: 10.1002/mrd.10317. Mol Reprod Dev. 2003. PMID: 12840810
-
Influence on spatiotemporal patterns of a male-specific Sox9 activation by ectopic Sry expression during early phases of testis differentiation in mice.Dev Biol. 2005 Feb 15;278(2):511-25. doi: 10.1016/j.ydbio.2004.11.006. Dev Biol. 2005. PMID: 15680367
-
The molecular action and regulation of the testis-determining factors, SRY (sex-determining region on the Y chromosome) and SOX9 [SRY-related high-mobility group (HMG) box 9].Endocr Rev. 2003 Aug;24(4):466-87. doi: 10.1210/er.2002-0025. Endocr Rev. 2003. PMID: 12920151 Review.
-
From SRY to SOX9: mammalian testis differentiation.J Biochem. 2005 Jul;138(1):13-9. doi: 10.1093/jb/mvi098. J Biochem. 2005. PMID: 16046443 Review.
-
Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds.Nat Genet. 1996 Sep;14(1):62-8. doi: 10.1038/ng0996-62. Nat Genet. 1996. PMID: 8782821
Cited by
-
SUMO represses transcriptional activity of the Drosophila SoxNeuro and human Sox3 central nervous system-specific transcription factors.Mol Biol Cell. 2005 Jun;16(6):2660-9. doi: 10.1091/mbc.e04-12-1062. Epub 2005 Mar 23. Mol Biol Cell. 2005. PMID: 15788563 Free PMC article.
-
The metastasis suppressor SOX11 is an independent prognostic factor for improved survival in gastric cancer.Int J Oncol. 2014 May;44(5):1512-20. doi: 10.3892/ijo.2014.2328. Epub 2014 Mar 6. Int J Oncol. 2014. PMID: 24604109 Free PMC article.
-
Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation.EMBO J. 2005 May 18;24(10):1798-809. doi: 10.1038/sj.emboj.7600660. Epub 2005 May 5. EMBO J. 2005. PMID: 15889150 Free PMC article.
-
Diverse functions of SOX9 in liver development and homeostasis and hepatobiliary diseases.Genes Dis. 2023 Jun 24;11(4):100996. doi: 10.1016/j.gendis.2023.03.035. eCollection 2024 Jul. Genes Dis. 2023. PMID: 38523677 Free PMC article. Review.
-
Inefficient Sox9 upregulation and absence of Rspo1 repression lead to sex reversal in the B6.XYTIR mouse gonad†.Biol Reprod. 2024 May 9;110(5):985-999. doi: 10.1093/biolre/ioae018. Biol Reprod. 2024. PMID: 38376238 Free PMC article.
References
-
- Sinclair A. H., Berta, P., Palmer, M. S., Hawkins, J. R., Griffiths, B. L., Smith, M. J., Foster, J. W., Frischauf, A. M., Lovell-Badge, R. & Goodfellow, P. N. (1990) Nature (London) 346, 240-244. - PubMed
-
- Gubbay J., Collignon, J., Koopman, P., Capel, B., Economou, A., Munsterberg, A., Vivian, N., Goodfellow, P. & Lovell-Badge, R. (1990) Nature (London) 346, 245-250. - PubMed
-
- Schmahl J., Eicher, E. M., Washburn, L. L. & Capel, B. (2000) Development (Cambridge, U.K.) 127, 65-73. - PubMed
-
- Buehr M., Gu, S. & McLaren, A. (1993) Development (Cambridge, U.K.) 117, 273-281. - PubMed
-
- Martineau J., Nordqvist, K., Tilmann, C., Lovell-Badge, R. & Capel, B. (1997) Curr. Biol. 7, 958-968. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

