Primary prevention of diclofenac associated ulcers and dyspepsia by omeprazole or triple therapy in Helicobacter pylori positive patients: a randomised, double blind, placebo controlled, clinical trial
- PMID: 12171952
- PMCID: PMC1773346
- DOI: 10.1136/gut.51.3.329
Primary prevention of diclofenac associated ulcers and dyspepsia by omeprazole or triple therapy in Helicobacter pylori positive patients: a randomised, double blind, placebo controlled, clinical trial
Abstract
Background: There is much controversy as to whether or not treatment of Helicobacter pylori reduces the occurrence of peptic ulcers during therapy with a non-steroidal anti-inflammatory drug (NSAID).
Aim: To assess the efficacy of triple therapy or omeprazole on the occurrence of diclofenac associated ulcers in H pylori positive patients.
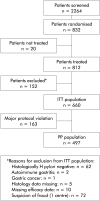
Methods: This was a randomised, double blind, placebo controlled, multicentre trial in H pylori positive patients requiring NSAID therapy who had no past or current peptic ulcer. They received diclofenac 50 mg twice daily for five weeks in combination with one of the four randomly assigned treatments: anti-H pylori treatment for one week (omeprazole 20 mg+clarithromycin 500 mg+amoxicillin 1 g, all twice daily) followed by placebo for four weeks (OAC-P); anti-H pylori treatment for one week followed by antisecretory treatment with omeprazole 20 mg once daily for four weeks (OAC-O); omeprazole 20 mg once daily for five weeks (O-O); or placebo for five weeks (P-P). Patients were endoscoped before and after treatment.
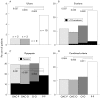
Results: Data from 660 patients were included in an intention to treat analysis. The occurrence of peptic ulcers in the four treatment groups during the study period was: 1.2% for OAC-P, 1.2% for OAC-O, 0% for O-O, and 5.8% for P-P (p<0.05 between placebo and all active treatment groups). Patients who received active treatment developed therapy requiring dyspeptic symptoms less frequently than those who received placebo (p<0.05 between placebo and all active treatment groups).
Conclusions: In H pylori infected patients, all three active therapies reduced the occurrence of NSAID associated peptic ulcer and dyspeptic symptoms requiring therapy.
Figures

Comment in
-
Helicobacter pylori and NSAID-associated ulcers.Am J Gastroenterol. 2004 Feb;99(2):397-8. doi: 10.1111/j.1572-0241.2004.04062.x. Am J Gastroenterol. 2004. PMID: 15046236 No abstract available.
References
-
- Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 1999;340:1888–99. - PubMed
-
- NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. Helicobacter pylori in peptic ulcer disease. JAMA 1994;272:65–9. - PubMed
-
- Laine L, Cominelli F, Sloane R, et al. Interaction of NSAIDs and Helicobacter pylori on gastrointestinal injury and prostaglandin production: a controlled double-blind trial. Aliment Pharmacol Ther 1995;9:127–35. - PubMed
-
- McCarthy CJ, Crofford LJ, Greenson J, et al. Cyclooxygenase-2 expression in gastric antral mucosa before and after eradication of Helicobacter pylori infection. Am J Gastroenterol 1999;94:1218–23. - PubMed
-
- Jones-Blackett S, Hull MA, Davies GR, et al. Non-steroidal anti-inflammatory drugs inhibit Helicobacter pylori-induced human neutrophil reactive oxygen metabolite production in vitro. Aliment Pharmacol Ther 1999;13:1653–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
