Disruption of pre-TCR expression accelerates lymphomagenesis in E2A-deficient mice
- PMID: 12172006
- PMCID: PMC123255
- DOI: 10.1073/pnas.162373999
Disruption of pre-TCR expression accelerates lymphomagenesis in E2A-deficient mice
Abstract
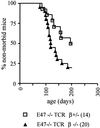
The helix-loop-helix proteins E47 and E12, which are encoded by the E2A gene, regulate several stages of T cell development. In addition, mice deficient for E2A are highly susceptible to thymic lymphoma. Here we report that the development of lymphoma in E2A-deficient mice did not require pre- and recombinase-activating gene expression. Rather, we found that, whereas illegitimate DNA rearrangement did not play a major role in the development of these lymphomas, defects that prevented pre-T cell antigen receptor expression tended to accelerate lymphomagenesis in E2A-deficient mice. These data and previous observations also provide insight into the role of Notch in lymphoma development. Specifically, we propose that Notch activation indirectly modulates E2A activity through induction of pre-Talpha expression, ultimately leading to the development of lymphoma.
Figures



Similar articles
-
E2A proteins enforce a proliferation checkpoint in developing thymocytes.EMBO J. 2004 Jan 14;23(1):202-11. doi: 10.1038/sj.emboj.7600017. Epub 2003 Dec 11. EMBO J. 2004. PMID: 14685278 Free PMC article.
-
Ectopic expression of E47 or E12 promotes the death of E2A-deficient lymphomas.Proc Natl Acad Sci U S A. 1999 Feb 2;96(3):996-1001. doi: 10.1073/pnas.96.3.996. Proc Natl Acad Sci U S A. 1999. PMID: 9927682 Free PMC article.
-
Early thymocyte development is regulated by modulation of E2A protein activity.J Exp Med. 2001 Sep 17;194(6):733-45. doi: 10.1084/jem.194.6.733. J Exp Med. 2001. PMID: 11560990 Free PMC article.
-
Functions of E2A-HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB.Mol Cell Biol. 2000 Sep;20(18):6677-85. doi: 10.1128/MCB.20.18.6677-6685.2000. Mol Cell Biol. 2000. PMID: 10958665 Free PMC article.
-
Thymocyte maturation is regulated by the activity of the helix-loop-helix protein, E47.J Exp Med. 1999 Dec 6;190(11):1605-16. doi: 10.1084/jem.190.11.1605. J Exp Med. 1999. PMID: 10587351 Free PMC article.
Cited by
-
Id1 expression promotes T regulatory cell differentiation by facilitating TCR costimulation.J Immunol. 2014 Jul 15;193(2):663-672. doi: 10.4049/jimmunol.1302554. Epub 2014 Jun 11. J Immunol. 2014. PMID: 24920844 Free PMC article.
-
E2A proteins enforce a proliferation checkpoint in developing thymocytes.EMBO J. 2004 Jan 14;23(1):202-11. doi: 10.1038/sj.emboj.7600017. Epub 2003 Dec 11. EMBO J. 2004. PMID: 14685278 Free PMC article.
-
The ID proteins: master regulators of cancer stem cells and tumour aggressiveness.Nat Rev Cancer. 2014 Feb;14(2):77-91. doi: 10.1038/nrc3638. Epub 2014 Jan 20. Nat Rev Cancer. 2014. PMID: 24442143 Review.
-
Oncogenesis of T-ALL and nonmalignant consequences of overexpressing intracellular NOTCH1.J Exp Med. 2008 Nov 24;205(12):2851-61. doi: 10.1084/jem.20081561. Epub 2008 Nov 3. J Exp Med. 2008. PMID: 18981238 Free PMC article.
-
E2A transcription factors limit expression of Gata3 to facilitate T lymphocyte lineage commitment.Blood. 2013 Feb 28;121(9):1534-42. doi: 10.1182/blood-2012-08-449447. Epub 2013 Jan 7. Blood. 2013. PMID: 23297135 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

