PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar
- PMID: 12172029
- PMCID: PMC151472
- DOI: 10.1105/tpc.003186
PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar
Erratum in
- Plant Cell 2002 Nov;14(11):2975
Abstract
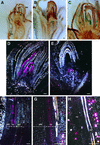
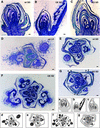
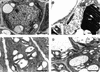
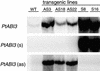
The Arabidopsis ABSCISIC ACID-INSENSITIVE3 (ABI3) protein plays a crucial role during late seed development and has an additional function at the vegetative meristem, particularly during periods of growth-arresting conditions and quiescence. Here, we show that the ABI3 homolog of poplar (PtABI3) is expressed in buds during natural bud set. Expression occurs clearly after perception of the critical daylength that initiates bud set and dormancy in poplar. In short-day conditions mimicking natural bud set, the expression of a chimeric PtABI3::beta-glucuronidase (GUS) gene occurred in those organs and cells of the apex that grow actively but will undergo arrest: the young embryonic leaves, the subapical meristem, and the procambial strands. If PtABI3 is overexpressed or downregulated, bud development in short-day conditions is altered. Constitutive overexpression of PtABI3 resulted in apical buds with large embryonic leaves and small stipules, whereas in antisense lines, bud scales were large and leaves were small. Thus, PtABI3 influences the size and ratio of embryonic leaves and bud scales/stipules that differentiate from the primordia under short-day conditions. These observations, together with the expression of PtABI3::GUS in embryonic leaves but not in bud scales/stipules, support the idea that wild-type PtABI3 is required for the relative growth rate and differentiation of embryonic leaves inside the bud. These experiments reveal that ABI3 plays a role in the cellular differentiation of vegetative tissues, in addition to its function in seeds.
Figures





References
-
- Bonetta, D., and McCourt, P. (1998). Genetic analysis of ABA signal transduction pathways. Trends Plant Sci. 3, 231–235.
-
- Crabbé, J.J. (1994). Dormancy. In Encyclopedia of Agricultural Science, Vol. 1, C.J. Arntzen and E.M. Ritter, eds (San Diego, CA: Academic Press), pp. 597–611.
-
- Dennis, F.G., Jr. (1996). A physiological comparison of seed and bud dormancy. In Plant Dormancy: Physiology, Biochemistry and Molecular Biology, G.A. Lang, ed (Wallingford, UK: CAB International), pp. 47–56.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

