Etanercept, infliximab, and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden
- PMID: 12176803
- PMCID: PMC1754224
- DOI: 10.1136/ard.61.9.793
Etanercept, infliximab, and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden
Abstract
Objective: To explore the feasibility of prospectively monitoring treatment efficacy and tolerability of infliximab, etanercept, and leflunomide over a two year period in patients with established rheumatoid arthritis (RA) in clinical practice using a structured protocol.
Methods: All patients with RA at seven centres in southern Sweden, for whom at least two disease modifying antirheumatic drugs, including methotrexate, had failed or not been tolerated, who started treatment with either infliximab, etanercept, or leflunomide were included. They were evaluated at predefined times using a standardised protocol including items required for evaluating response to the American College of Rheumatology (ACR) or EULAR criteria. All adverse events were recorded using World Health Organisation terminology. Concomitant treatment and survival while receiving a drug were recorded.
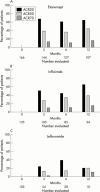
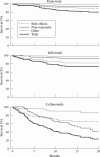
Results: During the study 166 patients were treated with etanercept, 135 with infliximab, and 103 with leflunomide. Treatment response as determined by the ACR and EULAR response criteria was similar for the tumour necrosis factor (TNF) blockers. The TNF blockers performed significantly better than leflunomide both as determined by the response criteria and by survival on drug analysis. Thus 79% and 75% continued to receive etanercept or infliximab compared with 22% of patients who started leflunomide after 20 months. The spectrum of side effects did not differ from those previously reported in the clinical trials. The initial two year experience of a protocol for postmarketing surveillance of etanercept, infliximab, and leflunomide shows that a structured protocol with central data handling can be used in clinical practice for documenting the performance of newly introduced drugs.
Conclusions: Efficacy data for the TNF blockers comply with results in clinical trials, whereas leflunomide appeared to perform worse than in clinical trials. Prolonged monitoring is required to identify possible rare side effects.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

