Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow
- PMID: 12177042
- PMCID: PMC2174028
- DOI: 10.1083/jcb.200204041
Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow
Abstract
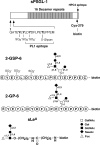
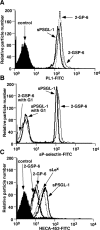
Leukocytes roll on selectins at nearly constant velocities over a wide range of wall shear stresses. Ligand-coupled microspheres roll faster on selectins and detach quickly as wall shear stress is increased. To examine whether the superior performance of leukocytes reflects molecular features of native ligands or cellular properties that favor selectin-mediated rolling, we coupled structurally defined selectin ligands to microspheres or K562 cells and compared their rolling on P-selectin. Microspheres bearing soluble P-selectin glycoprotein ligand (sPSGL)-1 or 2-glycosulfopeptide (GSP)-6, a GSP modeled after the NH2-terminal P-selectin-binding region of PSGL-1, rolled equivalently but unstably on P-selectin. K562 cells displaying randomly coupled 2-GSP-6 also rolled unstably. In contrast, K562 cells bearing randomly coupled sPSGL-1 or 2-GSP-6 targeted to a membrane-distal region of the presumed glycocalyx rolled more like leukocytes: rolling steps were more uniform and shear resistant, and rolling velocities tended to plateau as wall shear stress was increased. K562 cells treated with paraformaldehyde or methyl-beta-cyclodextrin before ligand coupling were less deformable and rolled unstably like microspheres. Cells treated with cytochalasin D were more deformable, further resisted detachment, and rolled slowly despite increases in wall shear stress. Thus, stable, shear-resistant rolling requires cellular properties that optimize selectin-ligand interactions.
Figures





References
-
- Alon, R., D.A. Hammer, and T.A. Springer. 1995. Lifetime of the P-selectin: carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 374:539–542. - PubMed
-
- Bell, G.I. 1978. Models for the specific adhesion of cells to cells: A theoretical framework for adhesion mediated by reversible bonds between cell surface molecules. Science. 200:618–627. - PubMed

