Human TPX2 is required for targeting Aurora-A kinase to the spindle
- PMID: 12177045
- PMCID: PMC2174010
- DOI: 10.1083/jcb.200204155
Human TPX2 is required for targeting Aurora-A kinase to the spindle
Abstract
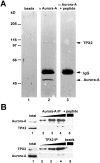
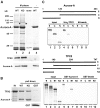
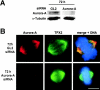
Aurora-A is a serine-threonine kinase implicated in the assembly and maintenance of the mitotic spindle. Here we show that human Aurora-A binds to TPX2, a prominent component of the spindle apparatus. TPX2 was identified by mass spectrometry as a major protein coimmunoprecipitating specifically with Aurora-A from mitotic HeLa cell extracts. Conversely, Aurora-A could be detected in TPX2 immunoprecipitates. This indicates that subpopulations of these two proteins undergo complex formation in vivo. Binding studies demonstrated that the NH2 terminus of TPX2 can directly interact with the COOH-terminal catalytic domain of Aurora-A. Although kinase activity was not required for this interaction, TPX2 was readily phosphorylated by Aurora-A. Upon siRNA-mediated elimination of TPX2 from cells, the association of Aurora-A with the spindle microtubules was abolished, although its association with spindle poles was unaffected. Conversely, depletion of Aurora-A by siRNA had no detectable influence on the localization of TPX2. We propose that human TPX2 is required for targeting Aurora-A kinase to the spindle apparatus. In turn, Aurora-A might regulate the function of TPX2 during spindle assembly.
Figures



References
-
- Bischoff, J., and G.D. Plowman. 1999. The Aurora/Ipl1p kinase family: regulators of chromosome segregation and cytokinesis. Trends Cell Biol. 9:454–459. - PubMed
-
- Boyle, W.J., G.P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110–149. - PubMed
-
- Elbashir, S., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 411:494–498. - PubMed
-
- Giet, R., and C. Prigent. 1999. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J. Cell Sci. 112:3591–3601. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

