Regulation of structural plasticity by different channel types in rod and cone photoreceptors
- PMID: 12177203
- PMCID: PMC6757881
- DOI: 10.1523/JNEUROSCI.22-16-07065.2002
Regulation of structural plasticity by different channel types in rod and cone photoreceptors
Abstract
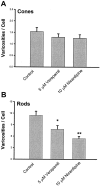
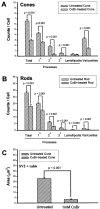
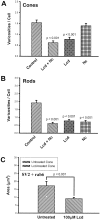
In response to retinal disease and injury, the axon terminals of rod photoreceptors demonstrate dramatic structural plasticity, including axonal retraction, neurite extension, and the development of presynaptic varicosities. Cone cell terminals, however, are relatively inactive. Similar events are observed in primary cultures of salamander photoreceptors. To investigate the mechanisms underlying these disparate presynaptic responses, antagonists to voltage-gated L-type and cGMP-gated channels, known to be present on rod and cone cell terminals, respectively, were used to block calcium influx during critical periods of plasticity in vitro. In rod cells, L-type channel antagonists nicardipine and verapamil inhibited not only the outgrowth of processes and the formation of varicosities, but also the synthesis of vesicle proteins, SV2 and synaptophysin. In contrast, the synthesis of opsin in rod cells was unaffected. In cone cells, L-type channel antagonists caused only modest changes. However, cobalt bromide, which blocks all calcium channels, and l-cis-diltiazem, a potent antagonist of cGMP-gated channels, significantly inhibited varicosity formation and synthesis of SV2 in cone cells. Moreover, the cGMP-gated channel agonist 8-bromo-cGMP caused a significant increase in varicosity formation by cone but not rod cells. Thus voltage-gated L-type channels in rod cells and cGMP-gated channels in cone cells are the primary calcium channels required for structural plasticity and the accompanying upregulation of synaptic vesicle synthesis. The differing responses of rod and cone terminals to injury and disease may be determined by these differences in the regulation of Ca2+ influx.
Figures









Similar articles
-
The nitric oxide-cGMP signaling pathway differentially regulates presynaptic structural plasticity in cone and rod cells.J Neurosci. 2005 Mar 9;25(10):2761-70. doi: 10.1523/JNEUROSCI.3195-04.2005. J Neurosci. 2005. PMID: 15758186 Free PMC article.
-
L-type calcium channels in the photoreceptor ribbon synapse: localization and role in plasticity.J Comp Neurol. 1999 Dec 6;415(1):1-16. J Comp Neurol. 1999. PMID: 10540354
-
Divalent cation selectivity is a function of gating in native and recombinant cyclic nucleotide-gated ion channels from retinal photoreceptors.J Gen Physiol. 1999 Jun;113(6):799-818. doi: 10.1085/jgp.113.6.799. J Gen Physiol. 1999. PMID: 10352032 Free PMC article.
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
-
Cyclic GMP-gated channel and peripherin/rds-rom-1 complex of rod cells.Novartis Found Symp. 1999;224:249-61; discussion 261-4. doi: 10.1002/9780470515693.ch14. Novartis Found Symp. 1999. PMID: 10614055 Review.
Cited by
-
EF hand-mediated Ca- and cGMP-signaling in photoreceptor synaptic terminals.Front Mol Neurosci. 2012 Feb 29;5:26. doi: 10.3389/fnmol.2012.00026. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22393316 Free PMC article.
-
The nitric oxide-cGMP signaling pathway differentially regulates presynaptic structural plasticity in cone and rod cells.J Neurosci. 2005 Mar 9;25(10):2761-70. doi: 10.1523/JNEUROSCI.3195-04.2005. J Neurosci. 2005. PMID: 15758186 Free PMC article.
-
Mislocalized opsin and cAMP signaling: a mechanism for sprouting by rod cells in retinal degeneration.Invest Ophthalmol Vis Sci. 2012 Sep 19;53(10):6355-69. doi: 10.1167/iovs.12-10180. Invest Ophthalmol Vis Sci. 2012. PMID: 22899763 Free PMC article.
-
Coming of Age for the Photoreceptor Synapse.Invest Ophthalmol Vis Sci. 2021 Sep 2;62(12):24. doi: 10.1167/iovs.62.12.24. Invest Ophthalmol Vis Sci. 2021. PMID: 34550300 Free PMC article.
-
Cone and rod cells have different target preferences in vitro as revealed by optical tweezers.Mol Vis. 2008 Apr 21;14:706-20. Mol Vis. 2008. PMID: 18432315 Free PMC article.
References
-
- Antony C, Cibert C, Géraud G, Santa Maria A, Maro B, Mayau V, Goud B. The small GTP-binding protein rab6 is distributed from medial Golgi to the trans-Golgi network as determined by a confocal microscopic approach. J Cell Sci. 1992;103:785–796. - PubMed
-
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel α1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–267. - PubMed
-
- Benfey M, Búnger UR, Vidal-Sanz M, Bray GM, Aguayo AJ. Axonal regeneration from GABAergic neurons in the adult rat thalamus. J Neurocytol. 1985;14:279–296. - PubMed
-
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
