Long-lasting sprouting and gene expression changes induced by the monoclonal antibody IN-1 in the adult spinal cord
- PMID: 12177206
- PMCID: PMC6757902
- DOI: 10.1523/JNEUROSCI.22-16-07097.2002
Long-lasting sprouting and gene expression changes induced by the monoclonal antibody IN-1 in the adult spinal cord
Abstract
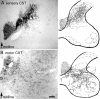
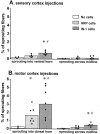
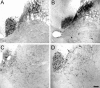
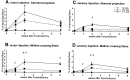
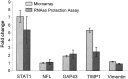
Lesion-induced plasticity of the rat corticospinal tract (CST) decreases postnatally, simultaneously with myelin appearance. In adult rats, compensatory sprouting can be induced by the monoclonal antibody (mAb) IN-1 raised against the growth inhibitory protein Nogo-A. In this study, we examined separately the fate of sensory and motor corticospinal fibers after mAb IN-1 application. Intact adult rats treated with the IN-1 antibody exhibited an increase of aberrant CST projections, i.e., sensory fibers projecting into the ventral horn and motor fibers projecting dorsally. Unilateral lesion of the CST [pyramidotomy (PTX)] in the presence of mAb IN-1 triggered a progressive reorganization of the sprouting of the remaining CST across the midline, with sensory fibers projecting gradually into the denervated dorsal horn and motor fibers projecting into the denervated ventral horn. In unilaterally denervated spinal cords, aberrant sprouts were only transient and disappeared by 6 weeks, whereas midline crossing fibers ending in the appropriate target region were stabilized and persisted over the entire study period. Within the spinal cord, IN-1 antibody treatment was associated with upregulation of growth factors (BDNF, VEGF), growth-related proteins (actin, myosin, GAP-43), and transcription factors (STATs), whereas pyramidotomy induced an enhanced expression of guidance molecules (semaphorins and slits) as well as neurotrophic factors (BDNF, IGFs, BMPs). These gene expression changes may contribute to attraction, guidance, and stabilization of sprouting CST fibers.
Figures


References
-
- Armand J. The origin, course and terminations of corticospinal fibers in various mammals. Prog Brain Res. 1982;57:329–360. - PubMed
-
- Bernstein DR, Stelzner DJ. Plasticity of the corticospinal tract following midthoracic spinal injury in the postnatal rat. J Comp Neurol. 1983;221:382–400. - PubMed
-
- Bregman BS, Kunkel-Bagden E, McAtee M, O'Neill A. Extension of the critical period for developmental plasticity of the corticospinal pathway. J Comp Neurol. 1989;282:355–370. - PubMed
-
- Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386:293–303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
