Specification of cerebellar progenitors after heterotopic-heterochronic transplantation to the embryonic CNS in vivo and in vitro
- PMID: 12177209
- PMCID: PMC6757871
- DOI: 10.1523/JNEUROSCI.22-16-07132.2002
Specification of cerebellar progenitors after heterotopic-heterochronic transplantation to the embryonic CNS in vivo and in vitro
Abstract
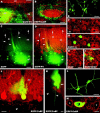
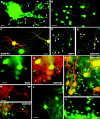
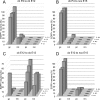
The different cerebellar phenotypes are generated according to a precise time schedule during embryonic and postnatal development. To assess whether the differentiative potential of cerebellar progenitors is progressively restricted in space and time we examined the fate of embryonic day 12 (E12) or postnatal day 4 (P4) cerebellar cells after heterotopic-heterochronic transplantation into the embryonic rat brain in utero or into organotypic CNS explants in vitro. Donor cells, isolated from transgenic mice overexpressing the enhanced-green fluorescent protein under the control of the beta-actin-promoter, engrafted throughout the host brainstem and diencephalon, whereas they rarely incorporated into specific telencephalic structures. In any recipient site, the vast majority of transplanted cells could be recognized as cerebellar phenotypes, and we did not obtain clear evidence that ectopically located cells adopted host-specific identities. Nevertheless, the two donor populations displayed different developmental potentialities. P4 progenitors exclusively generated granule cells and molecular layer interneurons, indicating that they are committed to late-generated cerebellar identities and not responsive to heterotopic-heterochronic environmental cues. In contrast, E12 precursors had the potential to produce all major cerebellar neurons, but the repertoire of adult phenotypes generated by these cells was different in distinct host regions, suggesting that they require instructive environmental information to acquire mature identities. Thus, cerebellar precursors are able to integrate into different foreign brain regions, where they develop mature phenotypes that survive long after transplantation, but they are committed to cerebellar fates at E12. Embryonic progenitors are initially capable, although likely not competent, to generate all cerebellar identities, but their potential is gradually restricted toward late-generated phenotypes.
Figures

Similar articles
-
Different types of cerebellar GABAergic interneurons originate from a common pool of multipotent progenitor cells.J Neurosci. 2006 Nov 8;26(45):11682-94. doi: 10.1523/JNEUROSCI.3656-06.2006. J Neurosci. 2006. PMID: 17093090 Free PMC article.
-
Engraftment and differentiation of neocortical progenitor cells transplanted to the embryonic brain in utero.J Neurocytol. 2004 May;33(3):309-19. doi: 10.1023/B:NEUR.0000044192.20397.7e. J Neurocytol. 2004. PMID: 15475686
-
Fate restriction and developmental potential of cerebellar progenitors. Transplantation studies in the developing CNS.Prog Brain Res. 2005;148:57-68. doi: 10.1016/S0079-6123(04)48006-6. Prog Brain Res. 2005. PMID: 15661181 Review.
-
Extracerebellar progenitors grafted to the neurogenic milieu of the postnatal rat cerebellum adapt to the host environment but fail to acquire cerebellar identities.Eur J Neurosci. 2010 Apr;31(8):1340-51. doi: 10.1111/j.1460-9568.2010.07167.x. Epub 2010 Apr 9. Eur J Neurosci. 2010. PMID: 20384777
-
Specification and differentiation of cerebellar GABAergic neurons.Cerebellum. 2012 Jun;11(2):434-5. doi: 10.1007/s12311-011-0324-8. Cerebellum. 2012. PMID: 22090364 Review.
Cited by
-
Development of cerebellar GABAergic interneurons: origin and shaping of the "minibrain" local connections.Cerebellum. 2008;7(4):523-9. doi: 10.1007/s12311-008-0079-z. Cerebellum. 2008. PMID: 19002744 Review.
-
Molecular layer interneurons of the cerebellum: developmental and morphological aspects.Cerebellum. 2015 Oct;14(5):534-56. doi: 10.1007/s12311-015-0648-x. Cerebellum. 2015. PMID: 25599913 Review.
-
Temporal identity transition from Purkinje cell progenitors to GABAergic interneuron progenitors in the cerebellum.Nat Commun. 2014;5:3337. doi: 10.1038/ncomms4337. Nat Commun. 2014. PMID: 24535035 Free PMC article.
-
Different types of cerebellar GABAergic interneurons originate from a common pool of multipotent progenitor cells.J Neurosci. 2006 Nov 8;26(45):11682-94. doi: 10.1523/JNEUROSCI.3656-06.2006. J Neurosci. 2006. PMID: 17093090 Free PMC article.
-
Cell-autonomous mechanisms and myelin-associated factors contribute to the development of Purkinje axon intracortical plexus in the rat cerebellum.J Neurosci. 2003 Jun 1;23(11):4613-24. doi: 10.1523/JNEUROSCI.23-11-04613.2003. J Neurosci. 2003. PMID: 12805301 Free PMC article.
References
-
- Alder J, Cho NK, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron. 1996;17:389–399. - PubMed
-
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure and functions. CRC; New York: 1997.
-
- Altman J, Das G. Autoradiographic and histological studies of postnatal histogenesis. II. A longitudinal investigation of kinetics, migration and transformation of cells incorporating thymidine in infant rat with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–390. - PubMed
-
- Alvarado-Mallart RM. Fate and potentialities of the avian mesencephalic/metencephalic neuroepithelium. J Neurobiol. 1993;24:1341–1355. - PubMed
-
- Anderson DJ. Stem cells and pattern formation in the nervous system: the possible versus the actual. Neuron. 2001;30:19–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
