Genetic influence on quantitative features of neocortical architecture
- PMID: 12177215
- PMCID: PMC6757858
- DOI: 10.1523/JNEUROSCI.22-16-07206.2002
Genetic influence on quantitative features of neocortical architecture
Abstract
The layout of functional cortical maps exhibits a high degree of interindividual variability that may account for individual differences in sensory and cognitive abilities. By quantitatively assessing the interindividual variability of orientation preference columns in the primary visual cortex, we demonstrate that column sizes and shapes as well as a measure of the homogeneity of column sizes across the visual cortex are significantly clustered in genetically related animals and in the two hemispheres of individual brains. Taking the developmental timetable of column formation into account, our data indicate a substantial genetic influence on the developmental specification of visual cortical architecture and suggest ways in which genetic information may influence an individual's visual abilities.
Figures





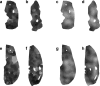


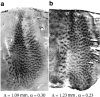


References
-
- Baaré WFC, Pol HEH, Boomsma DI, Posthuma D, de Geus EJC, Schnack HG, van Haren NEM, van Oel CJ, Kahn RS. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 2001;11:816–824. - PubMed
-
- Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120:257–269. - PubMed
-
- Blakemore C, Cooper GF. Development of the brain depends on the visual environment. Nature. 1970;228:477–478. - PubMed
-
- Bouchard TJ. Genetic and environmental influences on adult intelligence and special mental abilities. Hum Biol. 1998;70:257–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
