Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro
- PMID: 12177218
- PMCID: PMC6757904
- DOI: 10.1523/JNEUROSCI.22-16-07234.2002
Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro
Abstract
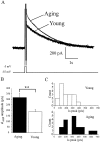
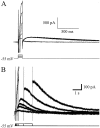
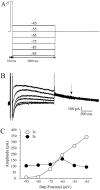
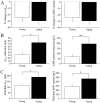
Aging is associated with learning deficits and a decrease in neuronal excitability, reflected by an enhanced post-burst afterhyperpolarization (AHP), in CA1 hippocampal pyramidal neurons. To identify the current(s) underlying the AHP altered in aging neurons, whole-cell voltage-clamp recording experiments were performed in hippocampal slices from young and aging rabbits. Similar to previous reports, aging neurons were found to rest at more hyperpolarized potentials and have larger AHPs than young neurons. Given that compounds that reduce the slow outward calcium-activated potassium current (sI(AHP)), a major constituent of the AHP, also facilitate learning in aging animals, the sI(AHP) was pharmacologically isolated and characterized. Aging neurons were found to have an enhanced sI(AHP,) the amplitude of which was significantly correlated to the amplitude of the AHP (r = 0.63; p < 0.001). Thus, an enhanced sI(AHP) contributes to the enhanced AHP in aging. No differences were found in the membrane resistance, capacitance, or kinetic and voltage-dependent properties of the sI(AHP). Because enhanced AHP in aging neurons has been hypothesized to be secondary to an enhanced Ca2+ influx via the voltage-gated L-type Ca2+ channels, we further examined the sI(AHP) in the presence of an L-type Ca2+ channel blocker, nimodipine (10 microm). Nimodipine caused quantitatively greater reductions in the sI(AHP) in aging neurons than in young neurons; however, the residual sI(AHP) was still significantly larger in aging neurons than in young neurons. Our data, in conjunction with previous studies showing a correlation between the AHP and learning, suggest that the enhancement of the sI(AHP) in aging is a mechanism that contributes to age-related learning deficits.
Figures


Similar articles
-
Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner.J Neurophysiol. 1992 Dec;68(6):2100-9. doi: 10.1152/jn.1992.68.6.2100. J Neurophysiol. 1992. PMID: 1491260
-
Ca(2+) channels involved in the generation of the slow afterhyperpolarization in cultured rat hippocampal pyramidal neurons.J Neurophysiol. 2000 May;83(5):2554-61. doi: 10.1152/jn.2000.83.5.2554. J Neurophysiol. 2000. PMID: 10805657
-
Voltage-clamp analysis of the potentiation of the slow Ca2+-activated K+ current in hippocampal pyramidal neurons.Hippocampus. 2000;10(2):198-206. doi: 10.1002/(SICI)1098-1063(2000)10:2<198::AID-HIPO9>3.0.CO;2-F. Hippocampus. 2000. PMID: 10791842
-
Age-related biophysical alterations of hippocampal pyramidal neurons: implications for learning and memory.Ageing Res Rev. 2002 Apr;1(2):181-207. doi: 10.1016/s1568-1637(01)00009-5. Ageing Res Rev. 2002. PMID: 12039438 Review.
-
Functional aspects of calcium-channel modulation.Clin Neuropharmacol. 1993;16 Suppl 1:S12-24. doi: 10.1097/00002826-199316001-00003. Clin Neuropharmacol. 1993. PMID: 8390916 Review.
Cited by
-
Physiology and pathology of calcium signaling in the brain.Front Pharmacol. 2012 Apr 13;3:61. doi: 10.3389/fphar.2012.00061. eCollection 2012. Front Pharmacol. 2012. PMID: 22518105 Free PMC article.
-
Control of IsAHP in mouse hippocampus CA1 pyramidal neurons by RyR3-mediated calcium-induced calcium release.Pflugers Arch. 2007 Nov;455(2):297-308. doi: 10.1007/s00424-007-0277-4. Epub 2007 Jun 12. Pflugers Arch. 2007. PMID: 17562071
-
Susceptibility to Calcium Dysregulation during Brain Aging.Front Aging Neurosci. 2009 Nov 27;1:2. doi: 10.3389/neuro.24.002.2009. eCollection 2009. Front Aging Neurosci. 2009. PMID: 20552053 Free PMC article.
-
Redox Signaling in Neurotransmission and Cognition During Aging.Antioxid Redox Signal. 2018 Jun 20;28(18):1724-1745. doi: 10.1089/ars.2017.7111. Epub 2017 May 31. Antioxid Redox Signal. 2018. PMID: 28467718 Free PMC article. Review.
-
Alterations in Intrinsic and Synaptic Properties of Hippocampal CA1 VIP Interneurons During Aging.Front Cell Neurosci. 2020 Oct 14;14:554405. doi: 10.3389/fncel.2020.554405. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33173468 Free PMC article.
References
-
- Andreasen M, Lambert JD. The excitability of CA1 pyramidal cell dendrites is modulated by a local Ca(2+)-dependent K(+)-conductance. Brain Res. 1995;698:193–203. - PubMed
-
- Bekkers JM. Distribution of slow AHP channels on hippocampal CA1 pyramidal neurons. J Neurophysiol. 2000;83:1756–1759. - PubMed
-
- Blanton MG, LoTurco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
