Transcription elongation factor Spt4 mediates loss of phosphorylated RNA polymerase II transcription in response to DNA damage
- PMID: 12177294
- PMCID: PMC134242
- DOI: 10.1093/nar/gkf475
Transcription elongation factor Spt4 mediates loss of phosphorylated RNA polymerase II transcription in response to DNA damage
Abstract
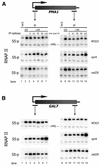
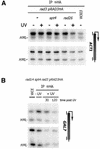
Previously, we found that Rad26, the yeast Cockayne syndrome B homolog and the transcription elongation factor Spt4 mediate transcription-coupled repair of UV-induced DNA damage. Here we studied the effect of DNA damage on transcription by directly analyzing the RNA polymerase II localization at active genes in vivo. A rad26 defect leads to loss of Ser5 phosphorylated RNA polymerase II localization to active genes, while localization is only transiently diminished in wild type cells. In contrast, loss of Ser5-P RNAP II localization is suppressed in spt4 cells. Interestingly, even when DNA damage is persistent the absence of Spt4 leads to a delayed loss of transcription suggesting that Spt4 is directly involved in mediating transcription shutdown. Comparative analysis of phosphorylated and non-phosphorylated RNA polymerase II localization revealed that Ser5-P RNAP II is preferentially lost in the presence of DNA damage. In addition, we found evidence for a transient Rad26 localization to active genes in response to DNA damage. These findings provide insight into the transcriptional response to DNA damage and the factors involved in communicating this response, which has direct implications for our understanding of transcription-repair coupling.
Figures



References
-
- Prakash S. and Prakash,L. (2000) Nucleotide excision repair in yeast. Mutat. Res., 451, 13–24. - PubMed
-
- de Laat W.L., Jaspers,N.G. and Hoeijmakers,J.H. (1999) Molecular mechanism of nucleotide excision repair. Genes Dev., 13, 768–785. - PubMed
-
- Mellon I., Spivak,G. and Hanawalt,P.C. (1987) Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell, 51, 241–249. - PubMed
-
- Selby C.P. and Sancar,A. (1990) Transcription preferentially inhibits nucleotide excision repair of the template DNA strand in vitro. J. Biol. Chem., 265, 21330–21336. - PubMed
-
- Selby C.P. and Sancar,A. (1993) Molecular mechanism of transcription-repair coupling. Science, 260, 53–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

