Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers
- PMID: 12177477
- PMCID: PMC166752
- DOI: 10.1104/pp.002337
Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers
Abstract
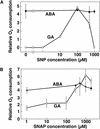
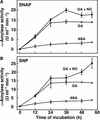
Nitric oxide (NO) is a freely diffusible, gaseous free radical and an important signaling molecule in animals. In plants, NO influences aspects of growth and development, and can affect plant responses to stress. In some cases, the effects of NO are the result of its interaction with reactive oxygen species (ROS). These interactions can be cytotoxic or protective. Because gibberellin (GA)-induced programmed cell death (PCD) in barley (Hordeum vulgare cv Himalaya) aleurone layers is mediated by ROS, we examined the effects of NO donors on PCD and ROS-metabolizing enzymes in this system. NO donors delay PCD in layers treated with GA, but do not inhibit metabolism in general, or the GA-induced synthesis and secretion of alpha-amylase. alpha-Amylase secretion is stimulated slightly by NO donors. The effects of NO donors are specific for NO, because they can be blocked completely by the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide. The antioxidant butylated hydroxy toluene also slowed PCD, and these data support our hypothesis that NO is a protective antioxidant in aleurone cells. The amounts of CAT and SOD, two enzymes that metabolize ROS, are greatly reduced in aleurone layers treated with GA. Treatment with GA in the presence of NO donors delays the loss of CAT and SOD. We speculate that NO may be an endogenous modulator of PCD in barley aleurone cells.
Figures







References
-
- Beligni MV, Lamattina L. Nitric oxide protects against cellular damage produced by methylviologen herbicides in potato plants. Nitric Oxide. 1999;3:199–208. - PubMed
-
- Beligni MV, Lamattina L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta. 2000;210:215–221. - PubMed
-
- Beligni MV, Lamattina L. Nitric oxide in plants: The history is just beginning. Plant Cell Environ. 2001;24:267–278.
-
- Bethke PC, Jones RL. Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. Plant J. 2001;25:19–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

