Organizational diversity among distinct glycoprotein endoplasmic reticulum-associated degradation programs
- PMID: 12181335
- PMCID: PMC117931
- DOI: 10.1091/mbc.e02-02-0068
Organizational diversity among distinct glycoprotein endoplasmic reticulum-associated degradation programs
Abstract
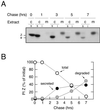
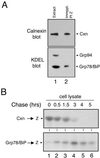
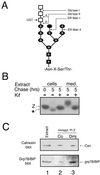
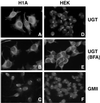
Protein folding and quality control in the early secretory pathway function as posttranslational checkpoints in eukaryote gene expression. Herein, an aberrant form of the hepatic secretory protein alpha1-antitrypsin was stably expressed in a human embryonic kidney cell line to elucidate the mechanisms by which glycoprotein endoplasmic reticulum-associated degradation (GERAD) is administered in cells from higher eukaryotes. After biosynthesis, genetic variant PI Z underwent alternative phases of secretion and degradation, the latter of which was mediated by the proteasome. Degradation required release from calnexin- and asparagine-linked oligosaccharide modification by endoplasmic reticulum mannosidase I, the latter of which occurred as PI Z was bound to the molecular chaperone grp78/BiP. That a distinct GERAD program operates in human embryonic kidney cells was supported by the extent of PI Z secretion, apparent lack of polymerization, inability of calnexin to participate in the degradation process, and sequestration of the glycoprotein folding sensor UDP-glucose:glycoprotein glucosyltransferase in the Golgi complex. Because UDP-glucose:glycoprotein glucosyltransferase sustains calnexin binding, its altered distribution is consistent with a GERAD program that hinders the reentry of substrates into the calnexin cycle, allowing grp78/BiP to partner with a lectin, other than calnexin, in the recognition of a two-component GERAD signal to facilitate substrate recruitment. How the processing of a mutant protein, rather than the mutation itself, can contribute to disease pathogenesis, is discussed.
Figures


References
-
- Ayalon-Soffer M, Shenkman M, Lederkremer GZ. Differential role of mannose and glucose trimming in the ER degradation of asialoglycoprotein receptor subunits. J Cell Sci. 1999;112:3309–3318. - PubMed
-
- Cabral CM, Choudhury P, Liu Y, Sifers RN. Processing by endoplasmic reticulum mannosidases partitions a secretion-impaired glycoprotein into distinct disposal pathways. J Biol Chem. 2000;275:25015–25022. - PubMed
-
- Cabral CM, Liu Y, Sifers RN. Dissecting glycoprotein quality control in the secretory pathway. Trends Biochem Sci. 2001;26:619–624. - PubMed
-
- Cannon KS, Helenius A. Trimming and re-addition of glucose to N-linked oligosaccharides determines calnexin association of a substrate glycoprotein in living cells. J Biol Chem. 1999;274:7537–7544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

