Function of dynein and dynactin in herpes simplex virus capsid transport
- PMID: 12181347
- PMCID: PMC117943
- DOI: 10.1091/mbc.01-07-0348
Function of dynein and dynactin in herpes simplex virus capsid transport
Abstract
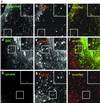
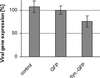
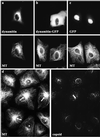
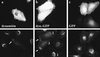
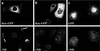
After fusion of the viral envelope with the plasma membrane, herpes simplex virus type 1 (HSV1) capsids are transported along microtubules (MTs) from the cell periphery to the nucleus. The motor ATPase cytoplasmic dynein and its multisubunit cofactor dynactin mediate most transport processes directed toward the minus-ends of MTs. Immunofluorescence microscopy experiments demonstrated that HSV1 capsids colocalized with cytoplasmic dynein and dynactin. We blocked the function of dynein by overexpressing the dynactin subunit dynamitin, which leads to the disruption of the dynactin complex. We then infected such cells with HSV1 and measured the efficiency of particle binding, virus entry, capsid transport to the nucleus, and the expression of immediate-early viral genes. High concentrations of dynamitin and dynamitin-GFP reduced the number of viral capsids transported to the nucleus. Moreover, viral protein synthesis was inhibited, whereas virus binding to the plasma membrane, its internalization, and the organization of the MT network were not affected. We concluded that incoming HSV1 capsids are propelled along MTs by dynein and that dynein and dynactin are required for efficient viral capsid transport to the nucleus.
Figures





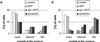
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

