TCL1 participates in early embryonic development and is overexpressed in human seminomas
- PMID: 12181493
- PMCID: PMC129334
- DOI: 10.1073/pnas.182412399
TCL1 participates in early embryonic development and is overexpressed in human seminomas
Abstract
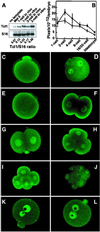
Overexpression of the TCL1 oncogene has been shown to play a causative role in T cell leukemias of humans and mice. The characterization of Tcl1-deficient mice in these studies indicates an important developmental role for Tcl1 in early embryogenesis. In wild-type embryos, Tcl1 is abundant in the first three mitotic cycles, during which it shuttles between nuclei and the embryo cortical regions in a cell-cycle-dependent fashion. The absence of this protein in early embryogenesis results in reduced fertility of female mice. The present studies elucidate the mechanism responsible for the reduced female fertility through analysis of the oogenesis stages and early embryo development in Tcl1-deficient mice. Even though Tcl1(-/-) females display normal oogenesis and rates of oocyte maturation/ovulation and fertilization, the lack of maternally derived Tcl1 impairs the embryo's ability to undergo normal cleavage and develop to the morula stage, especially under in vitro culture conditions. Beyond this crisis point, differentiative traits of zygotic genome activation and embryo compaction can take place normally. In contrast with this unanticipated role in early embryogenesis, we observed an overexpression of TCL1 in human seminomas. This finding suggests that TCL1 dysregulation could contribute to the development of this germinal cell cancer as well as lymphoid malignancies.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

