Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage
- PMID: 12181744
- PMCID: PMC4569095
- DOI: 10.1038/sj.cdd.4401048
Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage
Abstract
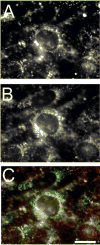
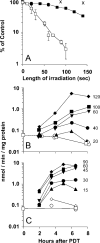
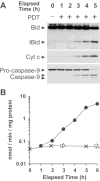
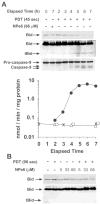
Photodynamic therapy (PDT) protocols employing lysosomal sensitizers induce apoptosis via a mechanism that causes cytochrome c release prior to loss of mitochondrial membrane potential (DeltaPsi(m)). The current study was designed to determine how lysosomal photodamage initiates mitochondrial-mediated apoptosis in murine hepatoma 1c1c7 cells. Fluorescence microscopy demonstrated that the photosensitizer N-aspartyl chlorin e6 (NPe6) localized to the lysosomes. Irradiation of cultures preloaded with NPe6 induced the rapid destruction of lysosomes, and subsequent cleavage/activation of Bid, pro-caspases-9 and -3. Pro-caspase-8 was not activated. Release of cytochrome c occurred at about the time of Bid cleavage and preceded the loss of DeltaPsi(m). Extracts of purified lysosomes catalyzed the in vitro cleavage of cytosolic Bid, but not pro-caspase-3 activation. Pharmacological inhibition of cathepsin B, L and D activities did not suppress Bid cleavage or pro-caspases-9 and -3 activation. These studies demonstrate that photodamaged lysosomes trigger the mitochondrial apoptotic pathway by releasing proteases that activate Bid.
Figures



References
-
- Agarwal ML, Clay ME, Harvey EJ, Evans HH, Antunex AR, Oleinick NL. Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res. 1991;51:5993–5996. - PubMed
-
- Oleinick NL, Morris RL, Belichenko I. Apoptosls in response to photodynamictherapy: what, where, why and how. Photochem Photobiol Sci. 2002;1:1–21. - PubMed
-
- Kessel D, Luo Y, Mathieu P, Reiners JJ., Jr Determinants of the apoptotic response to lysosomal photodamage. Photochem Photobiol. 2000;71:196–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

