Oxidative stress increases susceptibility of Mycobacterium tuberculosis to isoniazid
- PMID: 12183226
- PMCID: PMC127408
- DOI: 10.1128/AAC.46.9.2765-2771.2002
Oxidative stress increases susceptibility of Mycobacterium tuberculosis to isoniazid
Abstract
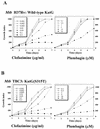
Isoniazid is a first-line antibiotic used in the treatment of infections caused by Mycobacterium tuberculosis. Isoniazid is a prodrug requiring oxidative activation by the catalase-peroxidase hemoprotein, KatG. Resistance to isoniazid can be obtained by point mutations in the katG gene, with one of the most common being a threonine-for-serine substitution at position 315 (S315T). The S315T mutation is found in more than 50% of isoniazid-resistant clinical isolates and results in an approximately 200-fold increase in the MIC of isoniazid compared to that for M. tuberculosis H37Rv. In the present study we investigated the hypothesis that superoxide plays a role in KatG-mediated isoniazid activation. Plumbagin and clofazimine, compounds capable of generating superoxide anion, resulted in a lower MIC of isoniazid for M. tuberculosis H37Rv and a strain carrying the S315T mutation. These agents did not cause as great of an increase in isoniazid susceptibility in the mutant strain when the susceptibilities were assessed by using the inhibitory concentration that causes a 50% decrease in growth. These results provide evidence that superoxide can play a role in isoniazid activation. Since clofazimine alone has antitubercular activity, the observation of synergism between clofazimine and isoniazid raises the interesting possibility of using both drugs in combination to treat M. tuberculosis infections.
Figures
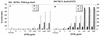



References
-
- Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. - PubMed
-
- Bosne-David, S., V. Barros, S. Cabo Verde, C. Portugal, and H. L. David. 2000. Intrisinic resistance of Mycobacterium tuberculosis to clarithromycin is effectively reversed by subinhibitory concentrations of cell wall inhibitors. J. Antimicrob. Chemother. 46:391-395. - PubMed
-
- Chinnaswamy, J., V. M. Reddy, and R. J. Gangadharam. 1995. Enhancement of drug susceptibility of multi-drug resistant strains of Mycobacterium tuberculosis by ethambutol and dimethyl sulfoxide. J. Antimicrob. Chemother. 55:381-390. - PubMed
-
- Cockerill, F. R., III, J. R. Uhl, Z. Temesgen, Y. Zhang, L. Stockman, G. D. Roberts, D. L. Williams, and B. C. Kline. 1995. Rapid identification of a point mutation of the Mycobacterium tuberculosis catalase-peroxidase (katG) gene associated with isoniazid-resistance. J. Infect. Dis. 171:240-245. - PubMed
-
- Deretic, V., E. Pogan-Ramos, Y. Zhang, S. Dhandayuthapani, and L. Via. 1996. The extreme sensitivity of Mycobacterium tuberculosis to the front-line antituberculosis drug isoniazid. Nat. Biotechnol. 14:1557-1561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

