The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro
- PMID: 12183260
- PMCID: PMC127409
- DOI: 10.1128/AAC.46.9.3001-3012.2002
The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro
Abstract
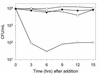
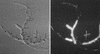
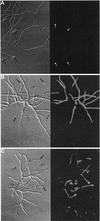
Caspofungin acetate is an antifungal antibiotic that inhibits synthesis of 1,3-beta-D-glucan, an essential component of the fungal cell wall. While caspofungin causes cell death in yeasts and dimorphic fungi such as Candida albicans, its effect on Aspergillus fumigatus is less well understood. We used the fluorescent dyes 5,(6)-carboxyfluorescein diacetate (CFDA) and bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC), which stain live and dead cells, respectively, to further characterize the antifungal activity of caspofungin. For comparison, compounds whose mode of action was either fungistatic (fluconazole, itraconazole) or fungicidal (amphotericin B) were also evaluated. A correlation between caspofungin-induced loss of viability, decreased CFDA staining, and increased DiBAC staining was established first with C. albicans. For A. fumigatus, caspofungin caused similar dye-staining changes, which were quantified by fluorimetric analysis of stained hyphae grown in a medium that promoted dispersed growth. The minimum concentration of caspofungin required to produce these changes also decreased the level of growth-dependent reduction of the indicator dye Alamar Blue. We observed a differential effect of caspofungin as a function of cell position: 88% of apical cells and 61% of subapical branching cells failed to stain with the viable dye CFDA, but only 24% of subapical cells were unstained. Complementary results were seen with germlings from DiBAC-stained, caspofungin-treated cultures. Extended incubation of A. fumigatus with a single dose of caspofungin affected the same proportion of apical and subapical branching cells for up to 72 h. The dye-staining patterns illustrate that the cells at the active centers for new cell wall synthesis within A. fumigatus hyphae are killed when they are exposed to caspofungin.
Figures
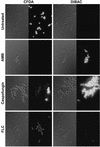

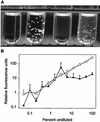
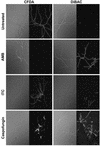
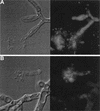

References
-
- Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, J. M. Balkovec, A. F. Bouffard, J. F. Dropinski, H. Rosen, H. Kropp, and K. Bartizal. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333-2338. - PMC - PubMed
-
- Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310-2318. - PMC - PubMed
-
- Anderson, J. G., and J. E. Smith. 1971. The production of conidiophores and conidia by newly germinated conidia of Aspergillus niger (microcycle conidiation). J. Gen. Microbiol. 69:185-197. - PubMed
-
- Archer, D. B., D. A. MacKenzie, and M. J. Ridout. 1995. Heterologous protein secretion by Aspergillus niger growing in submerged culture as dispersed or aggregated mycelia. Appl. Microbiol. Biotechnol. 44:157-160. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

