An N-terminal histidine regulates Zn(2+) inhibition on the murine GABA(A) receptor beta3 subunit
- PMID: 12183328
- PMCID: PMC1573463
- DOI: 10.1038/sj.bjp.0704835
An N-terminal histidine regulates Zn(2+) inhibition on the murine GABA(A) receptor beta3 subunit
Abstract
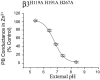
1. Whole-cell currents were recorded from Xenopus laevis oocytes and human embryonic kidney cells expressing GABA(A) receptor beta3 subunit homomers to search for additional residues affecting Zn(2+) inhibition. These residues would complement the previously identified histidine (H267), present just within the external portal of the ion channel, which modulates Zn(2+) inhibition. 2. Zinc inhibited the pentobarbitone-gated current on beta3(H267A) homomers at pH 7.4, but this effect was abolished at pH 5.4. The Zn(2+)-sensitive spontaneous beta3 subunit-mediated conductance was also insensitive to block by Zn(2+) at pH 5.4. 3. Changing external pH enabled the titration of the Zn(2+) sensitive binding site or signal transduction domain. The pK(a) was estimated at 6.8 +/- 0.03 implying the involvement of histidine residues. 4. External histidine residues in the beta3 receptor subunit were substituted with alanine, in addition to the background mutation, H267A, to assess their sensitivity to Zn(2+) inhibition. The Zn(2+) IC(50) was unaffected by either the H119A or H191A mutations. 5. The remaining histidine, H107, the only other candidate likely to participate in Zn(2+) inhibition, was substituted with various residues. Most mutants were expressed at the cell surface but they disrupted functional expression of beta3 homomers. However, H107G was functional and demonstrated a marked reduction in sensitivity to Zn(2+). 6. GABA(A) receptor beta3 subunits form functional ion channels that can be inhibited by Zn(2+). Two histidine residues are largely responsible for this effect, H267 in the pore lining region and H107 residing in the extracellular N-terminal domain.
Figures




References
-
- BARNARD E.A., DARLISON M.G., SEEBURG P.H. Molecular biology of the GABAA receptor: the receptor/channel superfamily. Trends in Neurosci. 1987;10:502–509.
-
- BLOOMENTHAL A.B., GOLDWATER E., PRITCHETT D.B., HARRISON N.L. Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+ Molec. Pharmacol. 1994;46:1156–1159. - PubMed
-
- CHANG Y., AMIN J., WEISS D.S. Zinc is a mixed antagonist of homomeric rho1 gamma-aminobutyric acid-activated channels. Molec. Pharmacol. 1995;47:595–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

