Distinct requirements for Ras oncogenesis in human versus mouse cells
- PMID: 12183360
- PMCID: PMC186434
- DOI: 10.1101/gad.993902
Distinct requirements for Ras oncogenesis in human versus mouse cells
Abstract
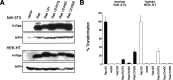
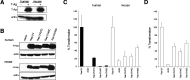
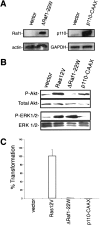
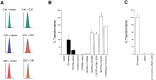
The spectrum of tumors associated with oncogenic Ras in humans often differs from those in mice either treated with carcinogens or engineered to sporadically express oncogenic Ras, suggesting that the mechanism of Ras transformation may be different in humans. Ras stimulates primarily three main classes of effector proteins, Rafs, PI3-kinase, and RalGEFs, with Raf generally being the most potent at transforming murine cells. Using oncogenic Ras mutants that activate single effectors as well as constitutively active effectors, we find that the RalGEF, and not the Raf or PI3-kinase pathway, is sufficient for Ras transformation in human cells. Thus, oncogenic Ras may transform murine and human cells by distinct mechanisms, and the RalGEF pathway--previously deemed to play a secondary role in Ras transformation--could represent a new target for anti-cancer therapy.
Figures


References
-
- Bergsagel DJ, Finegold MJ, Butel JS, Kupsky WJ, Garcea RL. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N Engl J Med. 1992;326:988–993. - PubMed
-
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
