The Drosophila heterochromatic gene encoding poly(ADP-ribose) polymerase (PARP) is required to modulate chromatin structure during development
- PMID: 12183365
- PMCID: PMC186441
- DOI: 10.1101/gad.1003902
The Drosophila heterochromatic gene encoding poly(ADP-ribose) polymerase (PARP) is required to modulate chromatin structure during development
Abstract
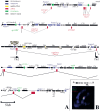
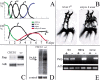
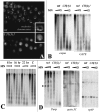
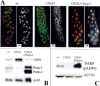
Poly(ADP-ribose) polymerase (PARP) is a major NAD-dependent modifying enzyme that mediates important steps in DNA repair, transcription, and apoptosis, but its role during development is poorly understood. We found that a single Drosophila Parp gene spans more than 150 kb of transposon-rich centromeric heterochromatin and produces several differentially spliced transcripts, including a novel isoform, PARP-e, predicted to encode a protein lacking enzymatic activity. An insertion mutation near the upstream promoter for Parp-e disrupts all Parp expression. Heterochromatic but not euchromatic sequences become hypersensitive to micrococcal nuclease, nucleoli fail to form, and transcript levels of the copia retrotransposon are elevated more than 50-fold; the variegated expression of certain transgenes is dominantly enhanced. Larval lethality can be rescued and PARP activity restored by expressing a cDNA encoding PARP-e. We propose that PARP-e autoregulates Parp transcription by influencing the chromatin structure of its heterochromatic environment. Our results indicate that Parp plays a fundamental role organizing the structure of Drosophila chromatin.
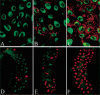
Figures


References
-
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. - PubMed
-
- Althaus FR. Poly ADP-ribosylation: a histone shuttle mechanism in DNA excision repair. J Cell Sci. 1992;102:663–670. - PubMed
-
- Althaus FR, Bachmann S, Hofferer L, Kleczkowska HE, Malanga M, Panzeter PL, Realini C, Zweifel B. Interactions of poly(ADPribose) with nuclear proteins. Biochimie. 1995;77:423–432. - PubMed
-
- Amé JC, Rolli V, Scureiber V, Niedergang C, Apiou F, Decker P, Muller S, Hoger T, Ménissier-de Murica J, de Murica G. PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J Biol Chem. 1999;274:17860–17868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
