Enhancement of protective efficacy following intranasal immunization with vaccine plus a nontoxic LTK63 mutant delivered with nanoparticles
- PMID: 12183520
- PMCID: PMC128246
- DOI: 10.1128/IAI.70.9.4785-4790.2002
Enhancement of protective efficacy following intranasal immunization with vaccine plus a nontoxic LTK63 mutant delivered with nanoparticles
Abstract
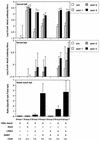
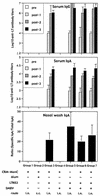
Most vaccines are still given parenterally. Mucosal vaccination would offer different advantages over parenteral immunization, including blocking of the pathogens at the portal of entry. In this paper, nontoxic Escherichia coli heat-labile enterotoxin (LT) mutants and Supramolecular Biovector systems (SMBV) were evaluated in mice as mucosal adjuvants and delivery systems, respectively, for intranasal immunization with the conjugated group C meningococcal vaccine. The conjugated vaccine formulated together with the LT mutants and the SMBV induced very high titers of serum and mucosal antibodies specific for the group C meningococcal polysaccharide. This vaccination strategy also induced high titers of antibodies with bactericidal activity, which is known to correlate with efficacy. Importantly, the mucosal vaccination, but not the conventional parenteral vaccination, induced bactericidal antibodies at the mucosal level. These data strongly support the feasibility of development of intranasal vaccines with an enhanced protective efficacy against meningococci and possibly against other encapsulated bacteria.
Figures

References
-
- Bartlett, G. R. J. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234:466-468. - PubMed
-
- Betbeder, D., A. Etienne, I. De Miguel, R. Kravtzoff, and M. Major. January 1995. Mucosal administration of substances to mammals. U.S. patent 6017513.
-
- Betbeder, D., C. Davrinche, J. L. Davignon, and E. Prieur. 1996. Method for enhancing immunogenicity product obtained and pharmaceutical composition. WO patent 96/06638.
-
- Castignolles, N., S. Morgeaux, C. Gontier-Jallet, D. Samain, D. Betbeder, and P. Perrin. 1996. A new family of carriers (biovectors) enhances the immunogenicity of rabies antigens. Vaccine 14:1353-1360. - PubMed
-
- Corthesy, B., and J. P. Kraehenbuhl. 1999. Antibody-mediated protection of mucosal surfaces. Curr. Top. Microbiol. Immunol. 236:93-112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

