Analysis of pathogen-host cell interactions in purpura fulminans: expression of capsule, type IV pili, and PorA by Neisseria meningitidis in vivo
- PMID: 12183570
- PMCID: PMC128269
- DOI: 10.1128/IAI.70.9.5193-5201.2002
Analysis of pathogen-host cell interactions in purpura fulminans: expression of capsule, type IV pili, and PorA by Neisseria meningitidis in vivo
Abstract
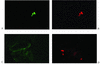
The pattern of meningococcal surface structure expression in different microenvironments following bloodstream invasion in vivo is not known. We used immunohistochemistry to determine the expression of capsule, type IV pili, and PorA by meningococci residing in the skin lesions of children with purpura fulminans. All the skin biopsy samples showed evidence of thrombosis and, frequently, a perivascular inflammatory cell infiltrate consisting of neutrophils (elastase positive) and monocytes/macrophages (CD68 positive). Modified Gram staining revealed 20 to over 100 gram-negative diplococci in each 4-microm-thick section, usually grouped into microcolonies. Immunoperoxidase staining demonstrated that the invading meningococci expressed PorA, capsule, and type IV pilin. Expression of these antigens was not restricted to any particular environment and was found in association with meningococci located in leukocytes, small blood vessels, and the dermal interstitium. Confocal laser scanning microscopy demonstrated coexpression of pilin and capsule by numerous microcolonies. However, there was some discordance in capsule and pilin expression within the microcolonies, suggesting phase variation. The strategy employed in this study will be helpful in investigating invasive bacterial diseases where antigenic and phase variation has a significant impact on virulence and on vaccine design.
Figures



References
-
- Bjerknes, R., H. K. Guttormsen, C. O. Solberg, and L. M. Wetzler. 1995. Neisserial porins inhibit human neutrophil actin polymerization, degranulation, opsonin receptor expression, and phagocytosis but prime the neutrophils to increase their oxidative burst. Infect. Immun. 63:160-167. - PMC - PubMed
-
- Bjune, G., E. A. Høiby, J. K. Grønnesby, Ø. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A.-K. Lindbak, H. Nøkleby, E. Rosenqvist, L. K. Solberg, O. Closs, J. Eng, L. O. Frøholm, A. Lystad, L. S. Bakketeig, and B. Hareide. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. - PubMed
-
- Brandtzaeg, P. 1995. Pathogenesis of meningococcal disease, p. 71-114. In K. Cartwright (ed.), Meningococcal disease. John Wiley & Sons Ltd., Chichester, United Kingdom.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

