Toll-like receptors: a family of pattern-recognition receptors in mammals
- PMID: 12186654
- PMCID: PMC139401
- DOI: 10.1186/gb-2002-3-8-reviews3011
Toll-like receptors: a family of pattern-recognition receptors in mammals
Abstract
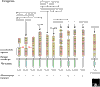
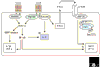
The innate immune system uses a variety of germline-encoded pattern-recognition receptors that recognize conserved microbial structures or pathogen-associated molecular patterns, such as those that occur in the bacterial cell-wall components peptidoglycan and lipopolysaccharide. Recent studies have highlighted the importance of Toll-like receptors (TLRs) as a family of pattern-recognition receptors in mammals that can discriminate between chemically diverse classes of microbial products. First identified on the basis of sequence similarity with the Drosophila protein Toll, TLRs are members of an ancient superfamily of proteins, which includes related proteins in invertebrates and plants. TLRs activate innate immune defense reactions, such as the release of inflammatory cytokines, but increasing evidence supports an additional critical role for TLRs in orchestrating the development of adaptive immune responses. The sequence similarity between the intracellular domains of the TLRs and the mammalian interleukin-1 and interleukin-18 cytokine receptors reflects the use of a common intracellular signal-transduction cascade triggered by these receptor classes. But more recent findings have demonstrated that there are in fact TLR-specific signaling pathways and cellular responses. Thus, TLRs function as sentinels of the mammalian immune system that can discriminate between diverse pathogen-associated molecular patterns and then elicit pathogen-specific cellular immune responses.
Figures
References
-
- Chuang T, Ulevitch RJ. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim Biophys Acta. 2001;1518:157–161. The cloning of the human TLR10 gene. - PubMed
-
- Du X, Poltorak A, Wei Y, Beutler B. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur Cytokine Netw. 2000;11:362–371. The identification of mouse TLR7, TLR8 and TLR9. - PubMed
-
- Chuang TH, Ulevitch RJ. Cloning and characterization of a subfamily of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw. 2000;11:372–378. The cloning and identifcation of human TLR7, TLR8 and TLR9. - PubMed
-
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. This is the first report showing a role for TLRs in mammalian immunity. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

