Surface cathepsin B protects cytotoxic lymphocytes from self-destruction after degranulation
- PMID: 12186841
- PMCID: PMC2196055
- DOI: 10.1084/jem.20011836
Surface cathepsin B protects cytotoxic lymphocytes from self-destruction after degranulation
Abstract
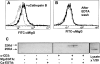
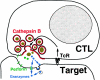
The granule exocytosis cytotoxicity pathway is the major molecular mechanism for cytotoxic T lymphocyte (CTL) and natural killer (NK) cytotoxicity, but the question of how these cytotoxic lymphocytes avoid self-destruction after secreting perforin has remained unresolved. We show that CTL and NK cells die within a few hours if they are triggered to degranulate in the presence of nontoxic thiol cathepsin protease inhibitors. The potent activity of the impermeant, highly cathepsin B-specific membrane inhibitors CA074 and NS-196 strongly implicates extracellular cathepsin B. CTL suicide in the presence of cathepsin inhibitors requires the granule exocytosis cytotoxicity pathway, as it is normal with CTLs from gld mice, but does not occur in CTLs from perforin knockout mice. Flow cytometry shows that CTLs express low to undetectable levels of cathepsin B on their surface before degranulation, with a substantial rapid increase after T cell receptor triggering. Surface cathepsin B eluted from live CTL after degranulation by calcium chelation is the single chain processed form of active cathepsin B. Degranulated CTLs are surface biotinylated by the cathepsin B-specific affinity reagent NS-196, which exclusively labels immunoreactive cathepsin B. These experiments support a model in which granule-derived surface cathepsin B provides self-protection for degranulating cytotoxic lymphocytes.
Figures
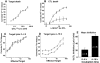





References
-
- Henkart, P.A. 1994. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1:343–346. - PubMed
-
- Harty, J.T., A.R. Tvinnereim, and D.W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275–308. - PubMed
-
- Stepp, S.E., R. Dufourcq-Lagelouse, F. Le Deist, S. Bhawan, S. Certain, P.A. Mathew, J.I. Henter, M. Bennett, A. Fischer, G. de Saint Basile, et al. 1999. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 286:1957–1959. - PubMed
-
- Straus, S.E., M. Sneller, M.J. Lenardo, J.M. Puck, and W. Strober. 1999. An inherited disorder of lymphocyte apoptosis: the autoimmune lymphoproliferative syndrome. Ann. Intern. Med. 130:591–601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

